- Submissions

Full Text
Environmental Analysis & Ecology Studies
Extraction of Oil and Phenolic Retanning Agent from Avocado Seed
Fasil Bereded Shiferaw*
School of Chemical and Bio Engineering, Ethiopia
*Corresponding author: Fasil Bereded Shiferaw, School of Chemical and Bio Engineering, Ethiopia
Submission: May 10, 2018; Published: January 23, 2019

ISSN 2578-0336 Volume4 Issue5
Abstract
Avocado seed oil is organic compounds consisting of both saturated and unsaturated fatty acids that can. The avocado seed was collected from the small fruit processing industry of municipal which discharges as waste to environment. The objective of this paper to develop retanning agent based on phenolic from avocado seed wastes for leather application and create eco-friendly environment. The avocado seed have two parts necessary for this work the oil and the tanning, due to this can be reused those part for other application. The method was followed by this project first to determine the physical properties of avocado seed like moisture content (61.5%), protein content (65.5), and ash content (1.43). And characterized the extracted oil of avocado seed like Acid value (7.73mg of KOH), Saponification value (214.5mg KOH/g), Ester value (207.3mg KOH), Iodine value (51.8mg iodine/g), Free Fatty Acid (3.63%) and then extracted the tannin based on the phenolic by using different solvent has gotten different result. Water based extracted tanning at 100 °C with 8hr was the optimized product for the leather application, but the hexane based extracted tanning was better given concentration of phenolic tannin.
The extracted tannin of avocado seed can be separated the liquor and the residual part then the liquor can be analysed with DLS for particle size determination and activity of the functional groups measured by FTIR. Based on this result the particle size of the extracted tannin was suitable for leather that can be determined by the exhaustion of the liquor. The optimized product applying in leather processing at stage of retanning process has shown a good leather physical test compare to the conventional processing.
keywordsAvocado seed; Avocado seed oil; Phenolic retanning agent; Solvent; Leather processing; Municipal waste; Eco-friendly
Introduction
Manufacturing of competent product of leather from raw hide and skin are a very complex course including lots of physical and chemical changes. On one hand, the useless parts are removed from raw hide to get necessary material for leather making of collagen fiber and opening the structure of collagen fiber. On the other hand, tanning agent are introducing to strengthen the stability of collagen fiber and other necessary materials are adding to make leather usable, such as fatliquoring, retanning and finishing agent. Moreover, different mechanical actions are needed in the course of those changes [1-8].
After retanning, the next stage is fatliquoring in order to lubricate the tanned fibers and fibrils with thin layers of oil and it is an essential operation in leather making. Fats and their derivatives are dispersible to impart the desired properties such as softness, feel, drape, run, suppleness, stretchiness, flexibility and additional strength to leather. Some of the essential physical properties like tensile strength and abrasion resistance are increased perceptibly by fatliquoring. It is the second last wet chemical step in the leather making process. Fatliquor impregnation of leather lubricates the fibers within leather, keeping them from adhering to each other. Since all leather factories apply chrome tanning, they use anionic type of fat liquor [9-15].
Avocado seed is by product of fruit processing industry and have a potential novel oil seed crop. Avocado seed oil also has many benefits, such as producing ecofriendly, biodiesel, paint and Studies in rats have shown that the oil from the avocado seed helps to increase the soluble collagen in the skin. As you age, your body naturally loses its ability to rebuild the collagen, but avocado seed oil helps to naturally restore it. Collagen helps to improve the overall tone of the skin by getting rid of wrinkles, dry flaky skin, cellulite and sclerosis of the skin [16-20].
Reducing cholesterol and fighting disease. It also contains many antioxidants which help you to feel great! By massaging avocado seed oil into your scalp, you will not only increase the growth of your hair, but it will come back to life with a new shine! Therefore, the seed oil is so popular in hand and body lotions, shampoos and other cosmetics; it simply helps you to look your very best from top to bottom.
However, the seed of the avocado is quite bitter, so you may not want to use it in your food. Just keep it handy for your cosmetic needs [21-28]. First of all, the fruit solid wastes should be characterized so that they can be reused. In this study, avocado seed wastes from different fruit juice industry have been analyzed with various chemical and instrumental analysis methods, and their characteristics have been defined. This data is thought to be useful in terms of preventing both environmental pollution and waste of resources by putting solid wastes into good use as secondary raw material in different industries rather than transferring them to disposal areas local fruit juice processing avocado seed as an alternative solution to current leather chemicals, the rise in price of which has had an adverse effect on the economy of the country [29-32].
Justification
Avocado seeds are normally wasting hence an alternative source of tanning (phenolic) and oil, for such process for cosmetics, starch, pharmaceutical and the polyphone for the syntan extraction. Utilization of seeds will greatly reduce space and money for waste disposal and management [33-43].
Material and Method
Raw materials, chemicals and reagents
Enough amount of avocado seed wastes was collected from local fruit juice processing located in Addis Ababa and Sheep wetblue for applied developed performance evaluation. All chemicals used in this study were analytical grade; analytical grade methanol (99%), hydrochloric Acid, Dichloromethane, Hexane, Sulfuric Acid, Sodium hydroxide and so on. All are accessed from leather industry development institute certified laboratory chemical stores in Addis Ababa [44-50].
Equipment and apparatus
Laboratory equipment and apparatus: The equipment used during the experimentations includes analytical weighing balance, Digital pH meter, Shaker, Crusher, Mechanical stirrer, Beaker, Burette, Measuring cylinder, Muffle furnace, Soxhlet extractor, Reflux condenser extractor, Micropipette, Water bath shaker, Sampling tube, Thermostats, Filter paper, Round bottom flask, Magnetic stirrer, Oven and desiccators, UV-Vis spectroscopy, FT-IR spectroscopy, Particle size analyser [51-57].
Leather processing equipment: Leather processing involve sample drum, testing drum, conditioning equipment, setting out machine staking machine, ball burst testing machine and tensile machine (Figure 1).
Figure 1:Frame work of the experiment.
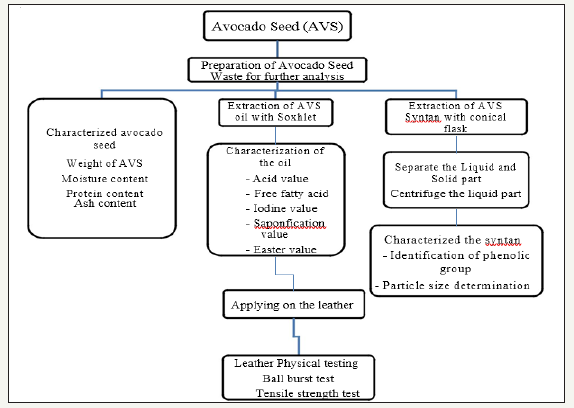
Method of experiment
Sampling and sample preparation: Enough amounts of avocado seed wastes were collected from local small fruit processing industry located in Addis Ababa. Avocado seeds served as a raw material for this study. The seeds were washed, and the outer covering of the seeds were manually removed while washing. The washed seeds were chopped and ambient condition air dried. For further drying, it was dried in an oven at 40 °C for 24hours. And ground into powder in a laboratory mill and were kept wrapped plastic bag [58-63].
Characterization of avocado seed: For the purpose of this study, avocado seed analyses the moisture content, seed weight, oil percentage, nitrogen, and ash content are determined.
Moisture content determination: Moisture content was determined using the procedure as follows: 0.5g of the milled sample was weighed using analytical balance, place in washed crucible and dried in a thermostatically controlled oven at 105 °C for 3hr. The sample was removed and placed in the desiccator and cooled to room temperature. The sample and the crucible were weighed repeatedly until a constant weight was obtained. Loss in weight of the sample was reported as moisture content Jorge, Gerardo, Díaz, February 2011.
Calculate the moisture content of the sample is calculated using the following equation:
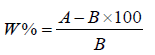
Where %W=Percentage of moisture in the sample,
A=Weight of wet sample (grams),
B=Weight of dry sample (grams),
Ash content determination: First measured the empty dried porcelain crucible, take 2.5grams of AVS powder sample was weighed into tared porcelain crucible and added 5ml of sulfuric acid then kept in the hood board until carbonized. It was ignited in a muffle furnace at 750 °C for 2hrs. Cooled in desiccator and weighed. The percent ash was determined using the following formula [4]
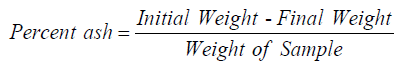
Crude protein determination: The Kjeldahl method was used to determine crude protein. 0.5g of the AVS flour sample, half of selenium-based catalyst tablet and a few anti-bumping agents were placed into a digestion flask. 25ml of concentrated sulphuric acid (H2SO4) was added to the sample and the flask shaken vigorously to obtain a wet and well mixed mixture. The flask was placed on a digestion burner and heated slowly until the boiling ceased and a clear solution was obtained. The solution was cooled to room temperature and the digested sample transferred into a 100ml volumetric flask. For distillation of the sample, the apparatus was flushed out before use. 25ml of 2% boric acid was pipetted into a 250ml conical flask and 2 drops of mixed indicator added.
Liquid was drained from the steam trap while leaving the stop cork which drains the steam trap opened. The conical flask with its content was placed under the condenser in a position where the tip of the condenser was completely immersed in the solution. 10ml of the digested sample was measured and added to the decomposition flask. 40% of NaOH (about 20ml) was also added to the decomposition flask. Distillation was allowed to continue for about 5minutes and the burner removed from the steam generator. The sample was titrated with 0.1N hydrochloric (HCl) solution until the sample solution became colourless.
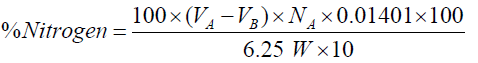
Where,
VA = Volume of standard acid (ml)
VB = Volume of standard acid in the blank (ml)
NA = Normality of HCl
W= Weight of sample (grams)
F(6.25) = Non-protein (urea) nitrogen factor
Extraction of oil: Soxhlet method was employed for the extraction of oil from avocado seed. Avocado seeds were obtained as a waste product from fruit processing industry with average moisture content of 47wt%. All solvents and chemicals obtained from Merk were used without any further purification. 25g weighed of the dried avocado seed samples moisture determination were transferred into six thimble the kept in chamber extraction unit of Soxhlet apparatus. A volume of 150ml of Petroleum, Hexane and Dichloromethane added 150ml was added respectively and apparatus assembled. Heated at a constant temperature of 100o C to reflux. The heat caused the solvent to vaporize through the thimble containing the sample as the solvent boiled in the flask; the vapor was trapped and cooled by the condenser above the thimble. The cooling turned the vapor into warm liquid which hydrolyzed the sample in the thimble. When the thimble was filled with the drops of the warm solvent from the condenser, the solvent (which contained traces of the oil) was poured out into the flat bottom flask beneath the thimble contain the solvent. The process was continued for the durations for 5hr [64-70].
At the end of each extraction process, the milled sample was removed from the thimble and the extraction process repeated, but this time for solvent recovery from the oil sample. This is done for an unspecified time depending on the quantity of oil and solvent contained in the flask from extraction. The oil was poured into a beaker and placed on a steam bath, and finally dried in the oven for 30minutes at 105 °C; the cooled in desiccators and weighed. The modern soxhlet apparatus can be assembled at the same temperature at 100 0c and adjust the running time (for extraction, rinsing, and drying). For this project work we were used three different solvent extraction (Petroleum, Haxane and DCM). The extraction was carried out using the electrical manual setup Soxhlet apparatus.
Chemical Characterization of Extracted Avocado Seed Oil
Determination of oil extracted
The maximum yield of avocado seed oil is determined under this formula. Its help for calculation of oil amount present in avocado seed [71-75].
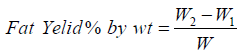
Where,
W1= Weight of the extraction flask (g)
W2=Weight of the extraction flask plus the dried crude fat (g)
W=Weight of sample (g)
Acid value (acid number)
The acid value (AV) is the number that expresses, in milligram the quantity of potassium hydroxide require to neutralize the free acids present in 1g of the substance (Annex 1).
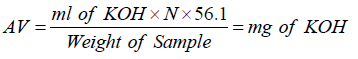
Where,
N=Normality of KOH
Free Fatty Acid (FFA)=AV×0.503
Calculate the acid value (AV) and free fatty acid (%FA) using above laws.
Saponification value
The saponification value is the number of mg potassium hydroxide required to neutralize the free acids and to saponify the ester in 1g of the substance. The saponification number is a measure of the average molecular weight of the triacylglycerols in sample. Saponification is the process of breaking down a neutral fat into glycerol and fatty acids by treatment with alkali. Saponification value is inversely proportional to mean molecular weight of fatty acid (or chain length). (annex 2)
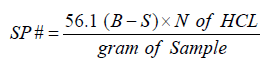
Where
B: ml of HCL required by blank
S: ml of HCL required by sample
Easter value
The easter value is defined as the mg of KOH required to react with glycerin (glycerol/ or glycerin) after saponify one gram of fat.
Iodine value (I.V)
The iodine value (IV) gives a measure of the average degree of unsaturation of a lipid: the higher iodine value, the greater the number of C=C double bonds. By definition the iodine value is expressed as the grams of iodine absorbed per 100g of lipid. Iodine value (I.V) is directly proportional to the degree of unsaturation (no of double bons.) and inversely proportional to the melting point (M.P) of lipid. An increase in I.V indicate high susceptibility of lipid to oxidative rancidity due to high degree of unsaturation.one of the most commonly used methods for determining the iodine value of lipid value of lipids is “Hanus Method”. The lipid to be analyzed is weighed and dissolved in a suitable organic solvent, to which a known excess of iodine chloride is added. Some of the IBr react with the double bonds in the unsaturated lipids, while the rest remains (annex3).
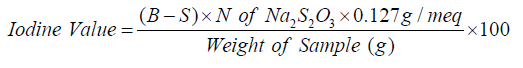
Where,
B: V ml of Na2S2O3 Volume for blank
S: V ml of Na2S2O3 volume for sample
Extraction of Syntan from the Waste of Avocado Seed
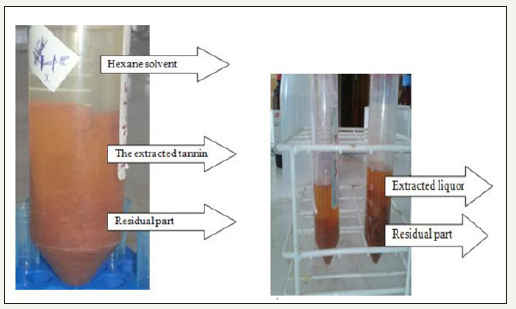
A sample of 20g of powdered avocado seeds was extracted with ratio 1:4 of distilled water in a conical flask at different temperature and time. Show the picture at Figure 2. The samples were heated at various temperatures of 70 °C, 80 °C, and 100 °C and time 4hr, 6hr and 8hr using a mantel hot plate. After the extraction, the extracts were then cooled and then filtered. Finally filtered liquor sample centrifuge at 25 °C with 8000rpm for 20min was subsequently used for the determination of total phenolic content (TPC) and another factional group.
Figure 2.

Characterization of the syntan
To characterize the properties of the syntan, the applied technique is determined the solid content, particle size, and checking the phenolic group and other functional group in AVS.
Determination of solid content
Take a 5ml of sample from the extracted liquor and transfer in to the crucible kept in oven at 105 °C for 3hr. cooled in the desiccator and weighed. The same thing done for the residual part of AVS. Until to get the constant weight. Then calculate the moisture content based on the Finally taking the value of constant by repeated it.
Determination Fourier Transform Infrared (FTIR) Spectroscopy Analysis
FT-IR spectroscopy was employed to acquire information on the chemical structure of the product sample. Through measurement and analysis of the resulting spectra analyze the functional group present in lignin can be distinguished, which help in identifying binding site (i.e., hydroxyl group) in syntan preparation stage. The spectrum of the sample was recorded on ABB MB3000 Fourier transform infra-red (FTIR) spectroscopy. All spectra were performed with the resolution of 4cm-1 and recorded at 45 °C incident angle using potassium bromide in the region 4000 to 6000cm-1.
Determination of particle size
The particle size was measured in DLS with high performance particle seizer (Zetasizer Nano series, Malvern) at 25 °C using the technique of photon correlation spectroscopy. With this technique the fluctuations in the intensity of light scattered by the particles were analyzed using a digital correlate to deter mine the diffusion coefficients. The diffusion coefficient is inversely proportional to the size of the particle and size was obtained from the Stokes Einstein equation. The obtained diffusion constant values were converted to intensity average particle size and number average particle size using CONTIN software employing Mie theory. The ions in the medium and the total ionic concentration can affect the particle diffusion speed by changing the thickness of the electric double layer called the Debye length (K-1). Thus, a low conductivity medium will produce an extended double layer of ions around the particle.
Application of the product in leather retanning
Conventional post tanning leather processing was applying for assisting the performance of residue of ASO based syntan and product will be comparing with leather develop using commercial phenolic syntan. The recipe can be shown annex
Physical test of leather
After the leathers are produced, it is necessary to test them to assess whether they will serve the ultimate purpose. As the properties of leather are affected by atmospheric temperature and varying humidity and as in the same place in different seasons of the year and the hour of the day, it is essential to conditions the leather, prior to testing, in a room under controlled conditions. The condition specified by the Indian Standard Specification are 20±2 °C and 60% R.H.±2 (R.H.=relative humidity) over a period of 48hrs. For leather this conditioning procedure is defined in ISO 2419 test method [51,52]. The conditioned samples are tested for various properties. The analyses for resistance were: tensile strength, elongation at break and tear load, in parallel (//) and perpendicular directions (⊥), the samples were analysed parallel and perpendicular to the dorsal line. The principals involved in testing various properties following with Official Standards methods are given below.
Tensile strength: The tensile strength was measured using Instron 1026 according to the official method (IUP/6, 2001). The samples were cut parallel and perpendicular to the backbone using a dumbbell shaped press knife. Each sample was measured in triplicate. The jaw of the tensile machine (Instron 1026) was set 50mm apart, and then the sample was clamped in the jaws, so that the edges of the Ove the rod, while the surface is watched for incipient cracking and bursting. The force and distention values at the point at which the grain side of the leather cracked and bursted was observed and the force and distention value recorded jaws lie along the mid line. The machine was run until the specimen broke and the highest load reached was taken as the breaking load. Tensile strength load is in Newton.
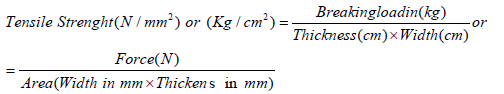
Elongation: The percentage elongation of leather is also a useful index of the stretching quality in many cases. The elongation is measured simultaneously with the measurement of tensile strength. Two reference marks are made in the narrow portion of the specimen before testing and the distance between these points is measured. The elongation also measures simultaneously with the tensile test of sample. The extension can be expressed as the percentage elongation at that load.
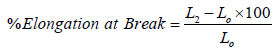
Where,
L2=Initial distance between the jaws in mm
Lo= final distance between the jaws in mm
Ball burst test: The ball burst test was measured using a lastometer according to the official method (IUP/9, 2001). A disc shaped specimen of the leather was firmly held with the grain side up between the clamping rings, with the spherical tip of the steel rod just touching the flesh surface. The specimen was moved downward against the rod, distending the grain of the leather immediately above the rod, while the surface is watched for incipient cracking and bursting. The force and distention values at the point at which the grain side of the leather cracked and bursted was observed and the force and distention value recorded.
Result and Discussion
Characterization of avocado seed
Determinations of moisture content of the avocado seed in average around 61.51% shown Table 1. During the chopping of the raw avocado seed was observed the color change from white to brownish. The picture has shown (Figure 2). This is the weight of Avocado seed in grams unit and taken 5 AVS counted numbers and weighed. The weight of avocado seed was determined using electronic weighing machine. The weight of avocado seed was given 42.3g. The nitrogen content of the avocado seed sample was determined and shown in Table 1. The % nitrogen content was used to calculate the protein content present in avocado seed. From the results obtained, the protein content of the avocado seed was calculated to be around 65.5
Table 1:Characterization of Avocado Seed (AVS).
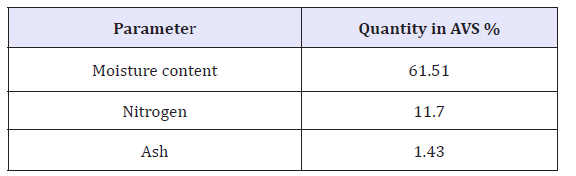
The mineral content of the avocado seed is very low it yields 1.43% so it implies that the avocado fruit is organic matter constitute and it can be degrading easily (Figure 3).
Figure 3:
Three layer of extracted tannin liquor after centrifuge.
3a. Hexane-based 3b. Water based
Identifying maximum yield of oil extraction from avocado seed
Determination of fat content and yield: The Oil content of avocado seed are determined and tabulated in Table 2. There were significant differences between mean yields oil extraction from avocado seed with solvents; dichloromethane, n-hexane and petroleum ether. It could be observed that the n-hexane solvent had given the maximum oil extractor of around 1.83 and DCM and PE had given 1.61% and 1.04%, respectively. Oil from sun drying is prepare sample is highly yield relatively with thermostatic oven drying, which can conclude that from cost wise sun drying is more preferable rather than using oven drying. Note: DCM (Dichlomethane), PE (pethroleume ether) and HE (n- hexane) (Table 2).
The result which obtained in the extraction of oil are influenced by a factor of solvent, finally the yield n- hexane are relatively better extracted than the other solvent. The Table 2 shows a percentage oil yield 1.59. The oil yield obtained in this study is however lower than 9.27±0.02% and 9.47±0.00% reported for unripe and ripe seeds of Persia Americana respectively in review.
Table 2:Extraction of oil from AVS with DCM, PE and HE.

Characterization of the extracted avocado seed oil: The physiochemical properties of avocado seed oil assayed in this study are presented in Table 3 shown below. The seed oil is a liquid at room temperature with a brownish-red color. The oil also has a strong characteristic fruity smell. According to FAO as reported by Akinoso and Raji (2010), seeds that contain oil yield greater than 17% are considered as oil seeds. The avocado pear seed is therefore not recommended for the purpose of edible oil generation and biodiesel production due to the very low oil yield. However, variation in oil yield may be due to the differences in species of plant, cultivation climate, ripening stage, the harvesting time of the seeds and the extraction method. as well as solvent used (Table 3).
Table 3:Results of oil characteristics.
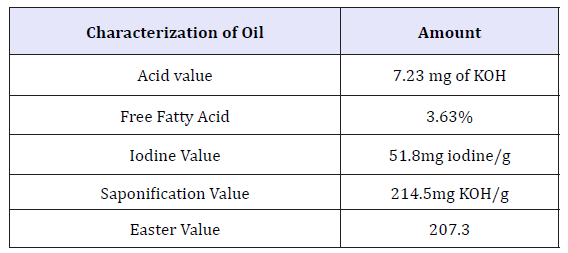
Acid value: Acid value is used to measure the extent to which glyceride in the oil has been decomposed by lipase and other actions such as light and heat. The lower the acid value of oil, the fewer free fatty acids it contains which makes it less susceptible to rancidity. According to [70], the lower the acid content, the more appealing the oil. Acid value of 7.23mg KOH/g was obtained for the avocado seed oil assayed in this study. This value is higher than the acid value which reported. The low acid value obtained for avocado seed oil in this study therefore suggests that the oil is edible and less susceptible to rancidity. The percentage free fatty acid (FFA) value of oil is a crucial parameter in determining the quality of oil because the lower the FFA, the higher the quality of the oil especially in terms of its edibility. The percentage free fatty acid of obtained 3.63% for avocado seed oil in this study is higher in comparison with reported. Low FFA content of the oil is also indicative of low susceptibility to enzymatic hydrolysis and could be an advantage over oils with high free fatty acids value which can become offflavor during storage [71].
Saponification value: Avocado seed oil had saponification values of 214.5 which is low in comparison previously reported. The relatively low saponification value of this oil may imply its poor suitability for the production of soaps and detergents.
Easter value: Ester value represents the number of milligrams of potassium hydroxide required to saponify the esters present in 1g of the oil. It is obtained as the difference between the saponification value and the acid value. Ester value of 207.3mg KOH/g was obtained for the avocado seed oil.
Iodine value: Iodine value suggests degree of unsaturation present in oil. Higher iodine value is attributed to high unsaturation. When compared with previously reported the iodine value 51.8mg iodine/g obtained for avocado seed oil in this work is low. This implies that the oil has relatively low degree of unsaturation and can thus be used as plasticizers and lubricants.
Characterization the Extracted Tannin from Avocado Seed
Experimental design was carried out to see the effects of temperature, solvent concentration and time in phenolic concentration. We were used different extract solvent hexane and water as solvent to raw material polyphenols avocado seed. Hexane concentration with the highest polyphenols yield compare to water. The time effect was measured between 4hr, 6hr and 8hr, the obtained result achieves the maximum number of polyphenols at higher time.
Temperature
Temperature bounds were taken at 70, 80 and 100 °C, to achieve the maximum temperature that obtained higher concentration of the polyphenol’s stability. All these parameters are collected in Table 4 which shows the experimental design for the variables temperature (T), hexane concentration, water concentration and time (t), with responses of polyphenolic, the particle size with Zeta potential (DLS) and functional groups measured by FTIR (Table 4).
Table 4:Tannin extraction from AVS with factor of time, temperature and solvent.
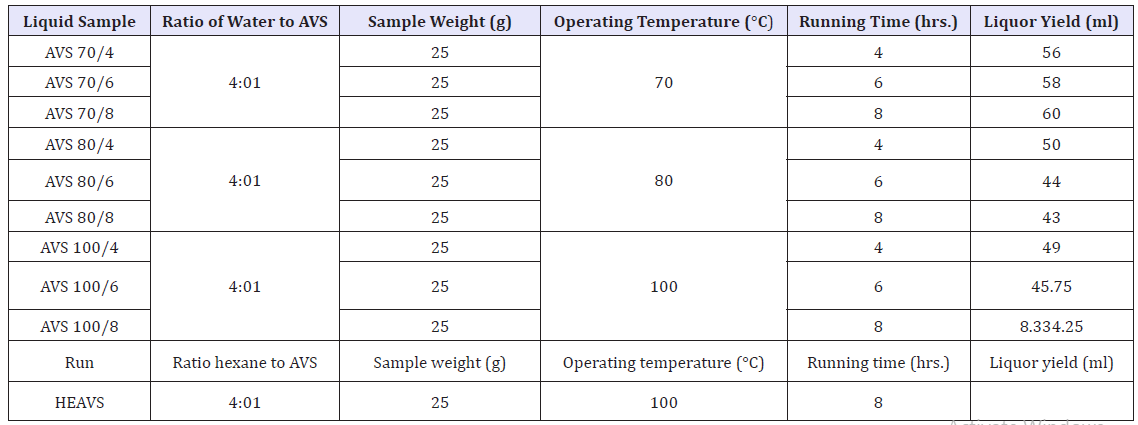
Appearance and factors of extracted tannin
During extracted tannin from avocado seed were rapidly converted from solid pieces of Avocado seed into a free flow liquid had shown Figure 4. The tannin extraction was carried out at different time; temperature and the effect of those factors. After extraction of hexane has shown three-layer formation (top hexane part, middle tannin and bottom some unextracted and residuals (Figure 4).
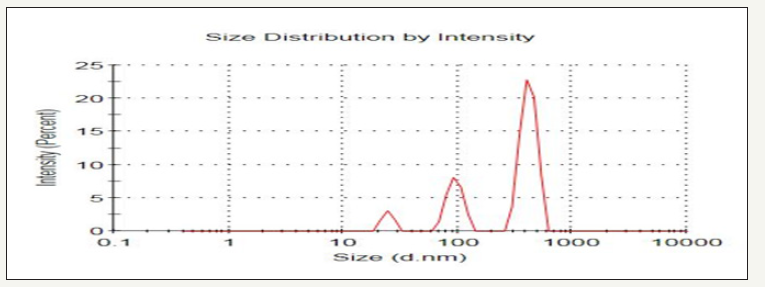
Figure 4:Graphical expression of the extracted tannin from Avocado seed of PSD (Paraticle size result of sample at 100 °C for 8hr).
Effect of temperature on extraction
The effect of temperature on the extraction of tannin of avocado seed was determined by maintaining the temperature at 70, 80 and 100 °C. Hence, the extracted temperature of 100 °C was fixed as optimum temperature.
Effect of time
The time of extraction plays a vital role for extraction of tannin from avocado seed samples. It could be observed that the sample after 8hours of extraction was viscous and was darker in color. On the other hand, 4hr has shown less concentration of tannin and obtained lighter color liquor. This could be attributed to the fact that with increased time of extraction, more amount of tannin got extracted and might result in higher concentration of phenolic groups.
Interpretation of particle size distribution
The PSD results were also shown in Figure 4. The graph below has been arranged using the dialogue to display a size distribution by Intensity graph as a histogram peak (Figure 4).
Analysis of FTIR
The FT-IR spectra of the synthesis product are given in Figure 5. The peak from 3400 cm-1 within the 3300 cm-1 absorption band confirms the presence of free phenolic groups has detected in the FTIR. The extracted tanning liquor power of avocado seed has shown in n-hexane has got a higher because of at Figure 5. The peak was shown sharp compare to water based extracted. In the case of solid (power of the extracted tanning) was obtained the maximum phenolic group relatively to the liquor of the tanning. The extracted at 100 °C with 8hr of the tanning avocado seed given the better phenolic concentration in extracted part compare to other graphical explanation of FTIR. In the case of fine solid has gotten by hexane the phenolic group had detected higher concentration of phenolic based on the graphical expression.
Figure 5:
The FT-IR spectra of the extracted tannin product liquor Avocado seed (a,b,c,d,e,f & g) and solid(s1, s2, s3 & s4).
Where S1: solid particle after extraction of oil,
S2: fine solid particle by hexane,
S3: solid particle by hexane (residual),
S4: liquid particle after denaturing by water then evaporate the moisture the solid part (@100 °C).

Determination of the solid content after centrifugation from the liquor of selected sample is found an average percentage value of 23.4%. Specifically, the result yields are greater at 100 °C for 8hr.
Application of developed products in leather processing
The extracted lubricated phenolic agents were used directly in the retanning stage. Two trials were carried out
A. Control process using commercial retanning agent
B. The extracted phenolic tannin product was used alone for upper leathers were developed using the process recipe provided.
For all the experiments, the leathers obtained had similar properties. The extracted phenolic tannin was penetrated in to the leather matrix without any problem. The dye exhaustion of the experimental leathers was better than the control leathers. Various physical analyses of the leathers were carried out as per standard procedures.
Physical testing of leather samples
The strength properties like tensile strength were tested using an instron tensile tester and grain crack & ball brust using lastometer of two matched side leather those were tested with preparing our extracted syntan and commercial synatn oil have been compared. The sheep upper leather was analyzed for tensile strength, % elongation at break, and bursting strength. The determined values are provided in below.
Tear strength: The tear strength of the leather provides an idea about strength of the leathers. The results are tabulated in Table 5. In the present study, leathers retanned with the extracted avocado tannin product gave highest value of 21.73%. This might be due to the fact that the leathers are fuller due to more phenolic fractions.
Table 5:Physical characteristics of control and experimental upper leathers.
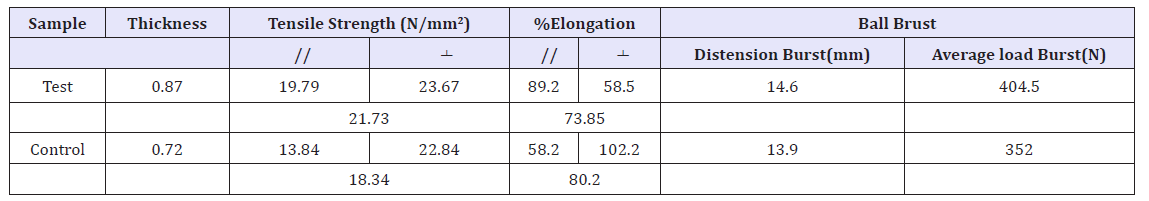
Ball burst (Distension and load crack): Shoe upper leather often shows slight crack in the toe area at the time of lasting operation in spite of the leather has good tensile strength properties. This is due to weak grain surface characteristics of leather due to more filling and loading of tanning and retanning materials in the grain side. The result of grain crack distension and load shown in Table 5. The extracted tanning was enhanced the ball burst test property of leather in retanning process.
Conclusion
This study focuses on the reutilization of avocado seed as beneficial product in leather processing after some extraction. Avocado seed are one of the most important by-products from fruit juice processing industry. Avocado seed have two part of fraction i.e. tannin and oil components. The oil was extracted from avocado seed obtained by dichloromethane (DCM), n-hexane (HE) and petroleum ether (PE) solvent and the tannin extraction of avocado seed was obtained by water base and hexane. The extracted tannin and oil from avocado seed was used for preparation of lubricant retanning agents and used in retannage of upper leathers.
The oil and the extracted tannin fractions were characterized and then used in the development of the product. Various parameters, such as time, temperature and solvent (concentration and amount) of tannin liquor have been optimized. The optimized product was used in the retanning of upper leathers. The results of the study showed that various parameters contributed to the extraction optimization of avocado seed oil as the n-hexane gave higher oil yields 1.59% as compared to other solvent (Table 6).
Table 6:Leather post tanning process recipe.
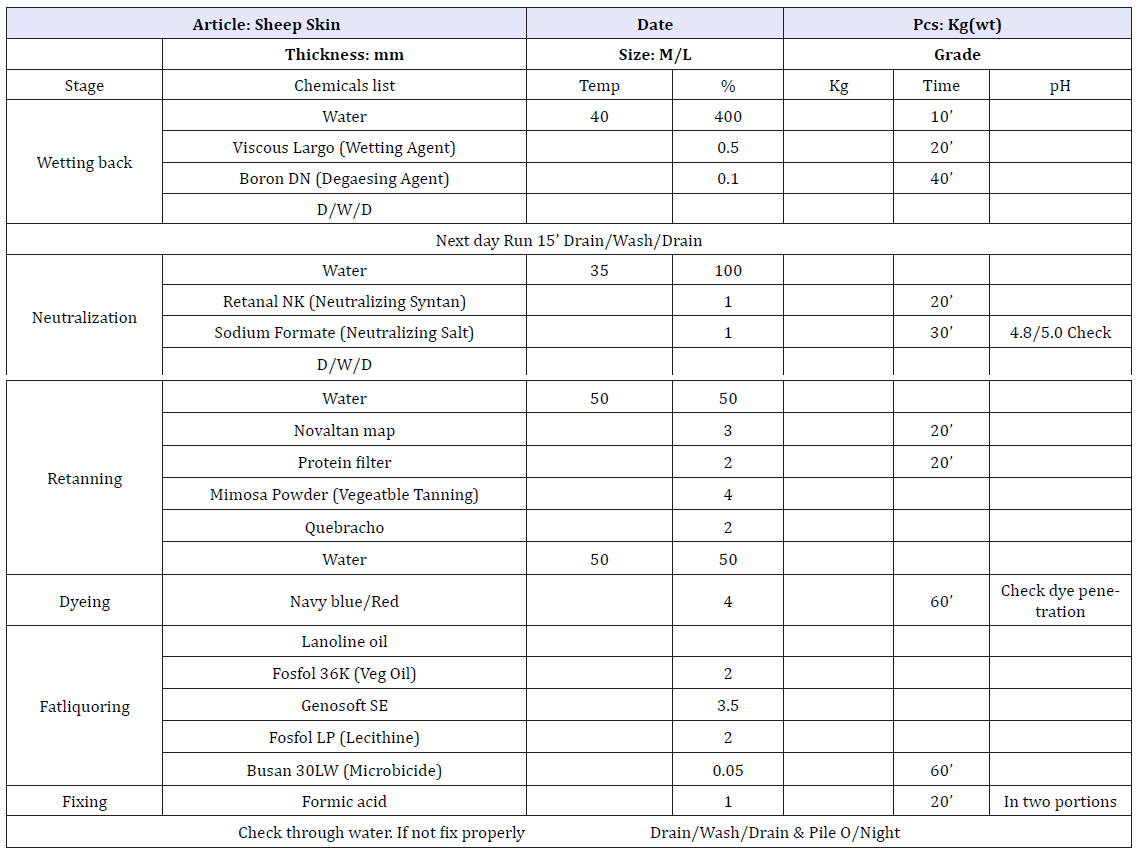
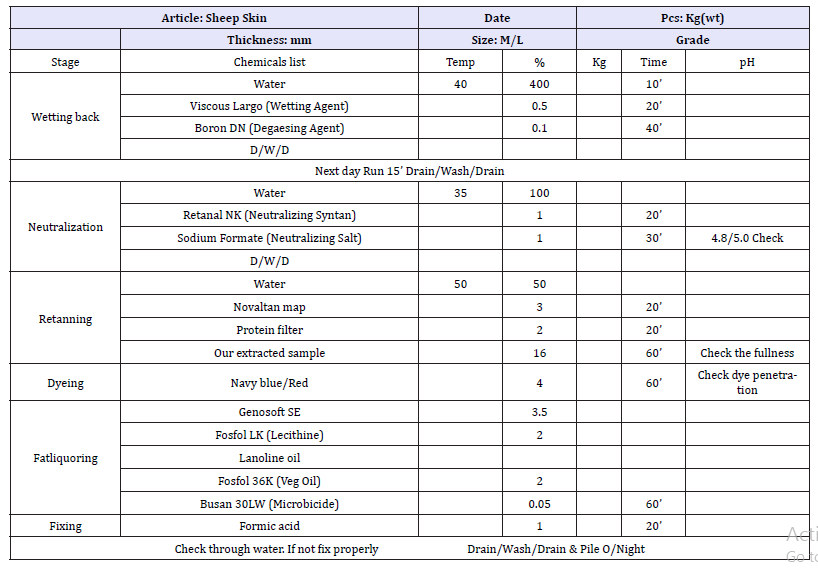
A. Sheep upper control process sheet.
According to the physicochemical analysis of the oil extract has been shown 3.63%, 7.73mg, 51.8mg iodine/g, 207.3mg KOH/g and 214.5mg KOH/g of Free fatty Acid (FFA), acid value (AV), Iodine value (IV), Ester value (EV) and Saponification value (SV). Literally there is an indication of the phenolic group are existed on the seed. To proof this we tried in different factor of solvent, time and temperature. The result of this is checked with FTIR analysis; from the result for 8hr at 100 °C is showed better resolution then other factors of extraction and also the fine solid power of hexane extracted (Table 7).
Table 7:For test sheep upper process sheet.
References
- Bienkiewicz KJ (1983) Physical chemistry of leather making. Robert E Krieger Publishing Company, Malabar, Florida, USA, p. 24.
- Pandarunga Rao S, Murgesan T, Suirianarayan M, Raghavan KV (1995) Chemical Engineering Science 50: 890.
- A Kumar CLRI kombolch tannery share company, training manual, under bench marking of technology up gradation, Ethiopia Herald Magazine, Ethiopia.
- Promising stride in harvesting, exporting avocado review on avocado value chain in ethiopia, Abduselam faris, Jimma University, Ethiopia.
- Nielson SS (1994) Introduction to the chemical analysis of foods. Chapman and Hall, New York, USA, pp. 93-207.
- Kirk RS, Sawyer R (1991) Pearson’s composition and analysis of foods, (9th edn), Addison Wesley Longman Ltd, England, pp. 9-29, 608-640.
- Wachsmann H (2001) Retanning or combination process. World Leather, pp. 64-65.
- Hauber C, Germann HP (1999) Investigation on a possible formation and voidance of chromate in leather. World Leather, pp. 73-80.
- Kiysztof Bieniewics (1983) Physical chemistry and leather making. In: Robert E Malabar (Ed.), Krieger Pub, Florida, USA, p. 541.
- Srearam KJ, Ramasami T (2003) Sustaining tanning process through conservation, recovery and better utilization of chromium. J Resources Conservation and Recycling 38(3): 185-212.
- Eid MA, Nashy EHA (2002) Speciation of chromium ions in tanning effluents and subsequent determination of Cr (VI) ICP-AES JALCA 97: 451.
- Anton El-Amma (1998) Structure property relationship of polyacrylate retanning agents. JALCA 93(1): 1-15.
- A El-A’mma, J Hodder, P Lesko (1991) A new lubricating acrylic syntan. JALCA 86: 1-7.
- Jing Hu, Jianzhong Ma, Weijun Deng (2008) Synthesis of alkali-soluble copolymer (butylacrylate/acrylic acid) and its application in leather finishing agent. European Polymer Journal 44(8): 2695-2701.
- Jing Hu, Jianzhong Ma, Weijun Deng (2008) Properties of acrylic resin/ nano-SiO2 leather finishing agent prepared via emulsifier-free emulsion polymerization. Materials Letters 62(17): 2931-2934.
- Mohamed OA, Moustafa AB, Mehawed MA, El-Sayed NH (2009) Styrene and butylmethacrylatecopolymers and their application in leather finishing. Journal of Applied Polymer Science 111(3): 1488-1495.
- Abd El-Ghaffar MA, El-Sayed NH, Masoud RA (2003) Modification of leather properties by grafting. I. Effect of monomer chain on the physicomechanical properties of grafted leather. Journal of Applied Polymer Science 896: 1478-1483.
- Klásek A, Kaszonyiová F Pavelka (2003) Grafting of 2-hydroxyethyl methacrylate and methyl methacrylate onto chrome tanned collagen fibers. Journal of Applied Polymer Science 31(7): 2007-2019.
- Klásek A, Imoníková J, Kaszonyiová A, Pavelka F, Janda L (2003) Grafting of 3-chloro-2-hydroxypropyl acrylate onto chrometanned collagen. Journal of Applied Polymer Science 31(7): 2021-2033.
- Madera-Sa TJ, Aguilar-Vega MJ, Alfredo Márquez‐Lucero F, Vázquez‐ Moreno (2002) Production of leather-like composites using chemically modified short leather fibers. (I): chemical modification by emulsion polymerization. Polymer Composites 23(1): 49-60.
- Ana V, Joana M, Sérgio B (2013) Physicochemical parameters, phytochemical composition and antioxidant activity of the algarvian avocado (persea americana mill). Journal of Agricultural Science 5(12): 100-109.
- Vinha AF, Barreira SVP, Castro A, Costa A, Oliveira MBPP (2013) Influence of the storage conditions on the physicochemical properties, antioxidant activity and microbial flora of different tomato (Lycopersicon esculentum) cultivars. Journal of Agricultural Science 5(2): 118-128.
- Deepti D, Ryan JE, Joshua DL, G Gregory RZ (2011) A colored avocado seed extract as a potential natural colorant. J Food Sci 76(9): C1335-C1341.
- Deshpande S, Salunke D (1982) Interaction of tannic acid and catechin with legume starches. Journal Food Science 47(6): 2080-2081.
- Rachimoellah HM, Dyah Ayu Resti, Ali Zibbeni, dan I Wayan Susil (2009) Production of biodiesel through transesterification of avocado (persea gratissima) seed oil using base catalyst. Journal of Mechanical Engineering 11(2): 85-90.
- Khanbabaee K, Van R (2001) Tannins: classification and definition, Nat. Prod Rep 18: 641-649.
- Haslam E (1989) Plant polyphenols, vegetable tannins revisited. Cambridge University Press, Cambridge, UK. pp. 64-100.
- Plavan V, Valeika V, Kovtunenko O, Širvaitytė J (2009) The pretreatment before tanning (Chrome or Non-Chrome). Journal of the Society of Leather Technologists and Chemists 93: 186-192.
- Jianzhong MA, Yun L, Bin L, Dangge G, Likun W (2009) Synthesis and properties of tannin/ vinyl polymer tanning agents.
- Reed R (2013) Science for students of leather technology, Pergamon press, Oxford, UK, pp. 30-240.
- Ogiwara C (1980) Practical guide to leather processing. Ferozsons printers Ltd., Tokyo, Japan, pp. 103-123.
- Faxing L, Yang L, Youjie H (2005) Preparation and the properties of vegetable extract used in low temperature tanning. Journal of Leather Science and Engineering 15(1): 22-26.
- Kaloka O, Moreki (2011) Tanning hides and skins using vegetable tanning agents in Hukuntsi sub-district, Botswana. Journal of Agricultural Technology 7(4): 915-922.
- Bryant JP, Reicharit BP, Clausen TP (1992) Chemically mediated interactions between woody plants and browsing mammals. Journal of Range Management 45: 18-27.
- Buelga, Williamson (2003) Methods in polyphenols analysis, The Royal Society of Chemist publisher, Cambridge, UK, pp. 1-5.
- Haroun M, Palmina K, Covington T (2013) Evaluation of vegetable tannin contents and polyphenols of some indigenous and exotic woody plant species in Sudan. Journal of Forest Products & Industries 2(4): 48-54.
- Slande D, Ferreira DJ, Marais JP (2005) Circular dichroism, a powerful tool for the assessment of absolute configuration of flavonoids. Photochemistry 66(18): 2177-2215.
- Murphy D J (1994) Designer oil crops. VCH Press, Weinheim, Germany.
- Rajagopal D, Khan A, Yoo KJ (2005) India’s unique sources of fuel for electricity and transportation funded by Mot-Unido program.
- NRI (Natural Resources Institute) (1995) Small scale vegetable oil extraction 5, 6, 7. p. 105.
- Sigmund R, Gustav E (1991) The cultivated plants of the tropics and subtropics. institute of agronomy in the tropic’s university. Pries GubH, Berlin, Germany, ISBN 3-8236-1169-0, pp. 76, 94-97.
- Khan LM, Hanna MA (1983) Expression of oil from oilseeds - A review. Journal of Agricultural Engineering Research 28(6): 495-503.
- Ali SB, Haroun HE, Musa AE (2013) Haraz bark powder extracts for manufacture of Nappa upper leather as alternatives retanning agents. Journal of Forest Production and Industries 2(5): 25-29.
- Grasser G (1992) Synthetic tannins. Enna FGA (Trans) p. 13.
- Flaherty FO, Roddy W and Lollar RM (1978) The chemistry and technology of leather. Robert E Krieger Publishing Company, Malabar, Florida, USA, pp. 387-458.
- Hemingway RW, Laks PE (1992) Plant polyphenols: synthesis, properties and significance. Springer publisher, pp. 30-40.
- Krause DO, Smith JM, Brouler JD, Sweeney CS (2005) Tolerance mechanism of streptococci to hydrolysable and condensed tannins. Journal of Animal Feed Science and Technologies 121(1-2): 59-75.
- Frutos P, Hervas G, Giraldez, Matelon A (2004) Review. Tanning and ruminant nutrition. Spanish Journal of Agricultural Research 2: 191-202.
- Sarkar KT (1995) Theory and practice of leather manufacturer. (4th edn), pp. 201-224.
- Reed R (2013) Science for students of leather technology, Pergamon press, Oxford, UK, pp. 30-24.
- Hergerman AE, Robbins CT, Weeradurigas XJ, Wilson TC, Arthur MC (1992) Tannins chemistry in relation to digestion. Journal of Range Management 45: 57-62.
- Hervey M, Allan B (1992) Tannins, their biochemistry and nutritional properties in plant cell. Biochemistry and Biotechnology, Vol 1, JAL press Ltd, London, UK, pp. 151-217.
- SLTC (1999) Society of Leather Technologist and Chemists Pocket book. SLTC publisher, East Yorkshire, UK, pp. 72-73.
- Kumar R, Singh M (1984) Tannins, their advance role in ruminant nutrition. Journal of Agricultural and Food Chemistry 32: 447-453.
- Covington AD (2009) Tanning chemistry: the science of leather. The Royal Society of Chemistry, Cambridge 10: 95-105.
- Thorstensen T (1993) Practical leather technology. (4th edn), Krieger publisher company, Malabar, Florida, USA, ISBN: 0-89464-689-3, pp. 147-171.
- Song Z, Williams CJ, Edyvean RG (2000) Sedimentation of Tannery Waste water. Water Research 34(7): 2171-2176.
- Wamegah R (2014) Vegetable tanning in Bolgatanga: Challenges and the way forward. Journal of Arts and Design Studies 16: 27-31.
- Musa AE, Gesmelseed GA (2012) Characterization of Lawsonia inermis as vegetable tanning materials. Journal of Forest Products and Industries 1(2): 35-40.
- CSA (2013) Agricultural sample survey 2012/2013; volume I, report on area and production of major crops, statistical bulletin 532; Addis Ababa, Ethiopia.
- Ayelech T (2011) Market chain analysis of fruits for Goma woreda Jimma zone, Oromia national regional state, A Thesis Submitted to School of Graduate Studies of Haramaya University, Ethiopia.
- Abduselam Faris (2016) Review on avocado value chain in ethiopia, Jimma University, Ethiopia.
- Zekarias Shumeta (2010) Avocado production and marketing in south western ethiopia. Journal of Trends in Agricultural Economics 3(4): 190- 206.
- Edossa Etissa (1997) Selection of avocado (Persea Americana) collection of desirable fruit characteristics and yield at jimma, proceedings of the 8th annual conference of the crop science society of ethiopia, Addis Ababa, Ethiopia, Education Ltd., London, pp. 26-35.
- Garedew W, Tsegaye B (2011) Trends of avocado (persea americana) production and its constraints in mana woreda, jimma zone: a potential crop for coffee diversification. Journal of Trends in Horticultural Research 1(1): 20-26.
- EHDA (2011) Exporting fruit and vegetable from Ethiopia. Reviewment of development potentials and investment options in the exportoriented fruit and vegetable sector. Addis Ababa, Ethiopia. p. 51.
- Berhanu M (2013) The role of avocado production in coffee-based farming systems of south western ethiopia. JASA 2(2): 86-95.
- Coenen JWE (1976) Hydrogenation of edible oils. Journal of the American oil Chemistry Society 53(6): 382-389.
- Bailey EA (1954) Industrial oil and fat products. (3rd edn), Interscience Publishers, London, UK.
- Deepalakshmi S, Sivalingam A, Thirumarimurugan M, Yasvanthrajan N, Sivakumar P (2014) In-situ transesterification and process optimization of biodiesel from waste avocado seed Composition of Avocado Seed. Journal of Chemical and Pharmaceutical Sciences, pp. 115-118.
- Rachimoellah HM, Dyah AR, Ali Zibbeni, Wayan Susila I Production of Biodiesel through Transesterification of Avocado (Persea gratissima) Seed Oil Using Base Catalyst 11(2): 85-90.
- HM Rachimoellah, Dyah Ayu Resti, Ali Zibbeni, dan I Wayan Susila chemical engineering, Faculty of Industrial Engineering, ITS, Surabaya, Indonesia.
- Sarkar KT (1995) Theory and practice of leather manufacturer. (4th edn), p. 776.
- Githinji P, Gitu L, Marete E, Githua M, Mugo M, Mbaka EN Quantitate analysis of total phenolic content in avocado (Persia American) seed in eastern province of KenyaJorge+Gerardo+Díaz.
- (2011) Development of sustainable tannin with low carbon foot print to obtain high quality leather, Muñoz, Barcelona, +February+2011 SLC 3 (IUC 5).
© 2019 Fasil Bereded Shiferaw. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






