- Submissions

Full Text
Environmental Analysis & Ecology Studies
Effect of Imidacloprid on Total Protein, Album in and Electrolytes in Heterobranchus Bidorsalis
Iniobong R Inyang, Sylvester C Izah* and Ntaka CM
Department of Biological Sciences, Nigeria
*Corresponding author: Sylvester C Izah, Department of Biological Sciences, Nigeria
Submission: April 29, 2018; Published: December 21, 2018

ISSN 2578-0336 Volume4 Issue5
Abstract
The aim of this study was to unveil the effects of imidacloprid (a pesticide) on some metabolites (albumin and total protein) and electrolytes (Na+, K+, Ca2+) in adult Heterobranchus bidorsalis (a common Niger wetland fish). Thirtyfive adult Heterobranchus bidorsalis (mean length, 22.43±2.42cm; mean weight, 166.70±0.33g) were acclimatized to laboratory condition for seven days and then exposed to varying sublethal concentrations of the toxicant (0.28, 0.42 and 0.56mgl-1) in a semi-static bioassay for 14 days. Albumin and total protein were determined in the liver while electrolytes were determined in the gastrointestinal tract (GIT). Results showed that total protein were 5.00μgl-1 at 0.00mg/l and 33.50μgl-1 at 0.56mg/l, albumin content were 4.00μgl-1 at 0.00mg/l and 13.00μgl-1 at 0.56mg/l, Sodium were 20.50mmoll-1at 0.00mg/l and 18.50mmoll-1 at 0.56mg/l , potassium 13.05 mmoll-1 at 0.00mg/l and 8.60 mmoll-1 at 0.56mg/l , and calcium were 0.25mmoll-1 at 0.00mg/l and 0.30 mmoll-1 at 0.56mg/l. There was significant variation (P< 0.05) among the various concentrations of the toxicant for each of the parameters except for calcium. Total protein and albumin values increased as the concentration of imidacloprid increased (in a dose dependent pattern). Sodium and potassium electrolytes values decreases down the experimental group, but not in dose dependent pattern. Albumin and total protein are useful biomarker of sub-lethal effects of imidacloprid than electrolytes. Additionally, the use of imidacloprid close to aquatic environment should be done with caution.
keywordsWQI; TSI; Use and land use; Water quality; Water resources
Introduction
Pesticide and other xenobiotics present in the aquatic environment can cause several physiological abnormalities and mortality in most organisms living in such ecosystem. Despite pesticide role in enhanced yields and productivity, it has negative ecological consequences in the environment [1]. Contamination of pesticides in the aquatic ecosystem can pose a serious threat to organisms like fish, which are exposed to most of these xenobiotics through anthropogenic depositions [2-11]. In most aquatic environment, studies have shown that fishes are the non-target organisms mostly affected by pesticides [2-7,12].Imidacloprid is a systemic chloronicotinyl insecticide that enters the target pest through injection or direct
contact [13]. Imidacloprid has the potential to disrupt nicotinic acetylcholine receptors in the insect central nervous system [14]. Typically, Imidacloprid is used in the treatment of seeds, soil, crops and control of domestic pests. According to Flores Céspedes et al. [15], imidacloprid affect non-target organisms such as honey bees, ground beetles and fishes. The authors further stated that, these organisms may adversely affect by sublethal doses of the insecticide, but the effect vary widely depending on application method and route of intake.
Contamination of water by pesticides either directly or indirectly can kill fish, reduced fish productivity or elevated concentration of undesirable toxicants in fresh water edible fish tissue which can greatly affect the health of humans consuming these fishes [16]. Fish responds to oxidative stress by evoking the enzymatic defense system within the body [17]. Pesticide are known to produce many physiological and biochemical changes in aquatic organisms by influencing the activities of several enzymes and metabolites [2-7,18]. Pesticide effect on electrolytes and total protein has been reported [3,4,9,18,19]. A decrease or increase in protein values as a result of exposure of fish to pesticide is an overt indication of physiological imbalance in the fish.
Biochemical indicators of environmental contamination such as enzymes, electrolytes and proteins may be sensitive and early warning indicators of short- or long-term detrimental effects of xenobiotics [20]. Metabolism can be described as the collective term for the chemical process that gives life [21]. Information on the energy metabolism and the factors that influence it is crucial to stress management and handling of fish [21]. The present study contributes to the assessment of Imidacloprid toxicity on some metabolites and electrolytes in Heterobranchus bidorsalis (a common Niger Delta wetland fish).
Materials and Methods
Experimental stock
Fish samples (adult Heterobranchus bidorsalis) for this study were obtained from a decent private farm at Yenagoa, Bayelsa State, Nigeria. They were transported to the wet laboratory of the Department of Biological Sciences, Niger Delta University, Bayelsa State, where the assays were conducted from January to March 2017. Thirtyfive (35) adult Heterobranchus bidorsalis (mean weight, 166.70±0.33g and mean length, 22.43±2.42cm) were acclimatized individually in a rectangular aquarium for seven days during which they were fed once a day (9.00-11.00hrs) with 35 crude protein at 1% biomass.
General bioassay techniques
Sublethal concentrations of imidacloprid (2.5EC) for the assay (0.28mg/l, 0.42mg/l, 0.56mg/l) were determined based on the range finding test [18]. These were prepared by transferring 0.33mls, 0.53mls, and 0.67mls respectively of the original concentration of the toxicant and making it up to 25L with borehole water on the test aquaria. 25L of the diluents (borehole water) was used as control. Fishes were introduced individually into each aquarium. The exposure period lasted for 14 days during which the exposure media were renewed every forty-eight hours. The physiochemical characterization of the water used for fish bioassay was carried out using standard methods [22] and the following values were obtained: Temperature (26.09-26.04 ̊C), pH (6.15- 6.19), alkalinity (13.35-15.19mgl-1), conductivity (.97-118μs/cm) and turbidity (0.49-0.53NTU).
After 14 days exposure period, fishes were killed for collection of samples for analysis via intestine (for electrolytes) and liver (for total protein and albumin). 0.5g of each sample was macerated (grounded) with pestle and mortar [23]. Samples for electrolytes were preserved in deionized water while metabolic samples were preserved using percloric acid. Samples were centrifuged at the rate of 3000rpm for 15 minutes, the supernatants were them removed and stored in plan bottles at-20 ̊C for analysis [23].
Metabolites and electrolytes analysis
Total protein and albumin were determined according to Tietz [24] and Doumas et al. [25] respectively, while APHA [22] method was used for all the electrolytes (Na+, K+, Ca2+).
Statistical analysis
The data were subjected to analysis of variance (ANOVA) where differences exist, Duncan multiple range test (DMRT) were used to test for pairwise significant difference (p< 0.05) between treatments [26].
Results and Discussion
Table 1 presents total protein and albumin in the liver of Heterobranchus bidorsalis exposed to imidacloprid for 14 days. The total protein was 5.00μgl-1 at 0.00mg/l, 10.25μgl-1 at 0.28mg/l, 20.50μgl-1 at 0.42 mg/l and 33.50μgl-1 at 0.56mg/l. There was significant elevation in total protein as the concentration of toxicant increased(Table 2). Typically, plasma proteins, which include globulins, fibrinogens and albumins, play essential role in transporting materials from one part of the fish to another via the circulation [18]. The findings of this report are contrary to the work of Inyang [18] that exposed Clarias gariepinus to diazinon (a wellknown organophosphate insecticide). The author also unveiled a gradation of values as the toxicant concentration increased. Increase in values of total protein is attributed to high energy demand in fish due to xenobiotics activities on the tissues of the fish. Due to the low carbohydrate level, protein which is the main architecture of fish cells and main source of nitrogenous metabolism is used to enhance energy demand [10,26,27]. The increase in protein concentration as the concentration of the toxicant increases suggests an interference in protein metabolism.
Table 1:Total protein and albumin in the liver of Heterobranchus bidorsalis exposed to imidacloprid for 14 days.
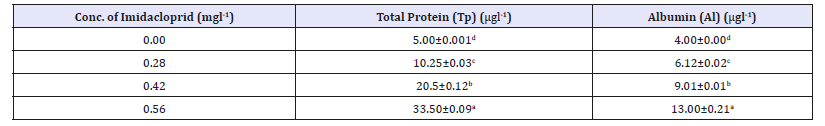
Means within column with different superscript are significantly different (p<0.05).
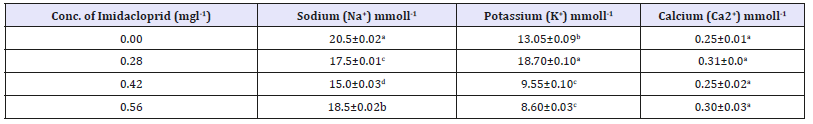
Table 2:Electrolytes (Sodium Na+), potassium (K+) and calcium (Ca2+) in the Gastro intestinal tract (GIT) of Heterobranchus bidorsalis exposed to imidacloprid for 14 days.
The albumin content was 4.00μgl-1 at 0.00mg/l, 6.12μgl-1 at 0.28mg/l, 9.01μgl-1 at 0.42mg/l and 13.00μgl-1 at 0.56mg/l, being significantly different (P< 0.05). Typically, there was significant elevation in albumin content as the concentration of toxicant increased. A rise in albumin values may be attributed to increase in protein synthesis due to increased enzyme activities involved in protein synthesis [28]. Typically, albumin plays essential role in exerting an osmotic potential which opposes the hydrostatic pressure developed in blood vessels [29]. Probably due to the antagonistic effect of the toxicant, albumin is needed by fish system for the removal of molecules such as calcium, bile, salts and some steroid hormones in the blood.
The electrolytes (calcium, sodium and potassium) showed fluctuation in the various concentration. Potassium and sodium values fluctuate down the experimental group (not in a dose dependent pattern). However, Sodium were 20.50mmoll-1 at 0.00mg/l and 18.50mmoll-1 at 0.56mg/l, potassium 13,05mmoll-1 at 0.00mg/l and 8.60mmoll-1 at 0.56mg/l, and calcium were 0.25mmoll-1 at 0.00mg/l and 0.30mmoll-1 at 0.56mg/l. There was significant variation (P< 0.05) in the electrolytes concentration except for calcium that were not significantly different (P>0.05).
The basic function of electrolytes in fishes lies in controlling fluid distribution, inter and extra cellular acido-basic equilibrium, maintaining osmotic pressure of the fluids and normal neuromuscular irritability [30]. Several authors have reported the effect of pesticides on fish electrolytes [5,6,9,18,23,31]. Stabilization in values (GIT calcium) could be a stress induced response of fish to toxicants which may have activated certain physiological and processes that could lead to a rapid uptake of the electrolyte from water, food material and a possible reduction of ion efflux [32]. Erhunmwuse & Aninerua [33] reported that sodium is the main regulator of osmotic pressure of the body fluid, and it also initiates and maintains the contraction of heart and involuntary muscles and excites the nerves. Sodium and potassium are essential for acid-base balance and osmotic pressure of the body, while potassium and calcium are essential for neuromuscular excitability [34], hence decline values of these electrolytes could impact on the cardiac roles of the fish system and general physiology since tissues, organs and systems work together for the general wellbeing of the organism [23].
Conclusion
We conclude that total protein and albumin in the liver could serve as a biomarker for evaluating effect of xenobiotics in Heterobranchus bidorsalis. Additionally, the use of this xenobiotics close to aquatic environment should be done with caution.
References
- Chindah AC, Sikoki FO, Vincent AJ (2004) Toxicity of an organophosphate pesticide (Chloropyrifos) on a common Niger Delta wetland fish. Tilapia genuineensis (Blecher 1962). Journal of Applied Science and Environmental Management 8(2): 11-17.
- Inyang IR, Ajimmy R, Izah SC (2017) Organosomatic index and behavioral response of heterobranchus bidorsalis exposed to rhonasate 360sl containing glyphosate (isopropylamine salt glycine). ASIO J Microbiol Food Sci Biotechnol Innov 3(1): 6-14.
- Inyang IR, Izah SC, Johnson DT, Ejomarie OA (2017) Effects of Lambda cyhalothrin on some electrolytes and metabolites in organs of Parpohiocephalus obscurus. Biotechnol Res 3(1): 6-10.
- Inyang IR, Ollor AO, Izah SC (2017) Effect of diazinon on organosomatic indices and behavioural responses of Clarias gariepinus (a Common Niger Delta Wetland Fish). Greener J Biol Sci 7(2): 15-19.
- Inyang IR, Seiyaboh EI, Job UB (2017) Condition factor, organosomatic indices and behavioural abnormalities of Clarias gariepinus exposed to lambda cyhalothrin. Greener J Life Sci 4(1): 001-005.
- Inyang IR, Akio K, Izah SC (2016) Effect of dimethoate on lactate dehydrogenase, creatinine kinase and amylase in Clarias lazera. Biotechnol Res 2(4): 155-160.
- Inyang IR, Kenobi A, Izah SC (2016) Effect of dimethoate on some selected metabolites in the brain, liver and muscle of Clarias lazera. Sky J Biochem Res 5(4): 63-68.
- Inyang IR, Obidiozo OZ, Izah SC (2016c) Effects of Lambda cyhalothrin on protein and Albumin content in the kidney and liver of Parpohiocephalus obscurus. EC Pharmacol Toxicol 2(3): 148-153.
- Inyang IR, Okon NC, Izah SC (2016) Effect of glyphosate on some enzymes and electrolytes in Heterobranchus bidosalis (a common African catfish). Biotechnol Res 2(4): 161-165.
- Inyang IR, Thomas S, Izah SC (2016) Activities of electrolytes in kidney and liver of Clarias gariepinus exposed to fluazifop-p-butyl. J Biotechnol Res 2(9): 68-72.
- Kumar NJI, Kumar RN, Bora A, Amb MK (2009) Photosynthetic, biochemical and enzymatic investigation of Anabaena fertilissima in response to an insecticide-hexachloro-hexahydromethanobenzodioxathiepine- oxide. J Journal of Stress Physiology & Biochemistry 5(3): 4-12.
- Inyang IR, Thomas S, Izah SC (2016) Evaluation of activities of transferases and phosphatase in plasma and organs of Clarias gariepinus exposed to Fluazifop-p-Butyl. J Environ Treat Techniq 4(3): 94-97.
- Meister RT (2000) Farm chemical handbook. (3rd edn), Meister publishers. Willoughby, OH, pp. 33-38.
- EPA (2005) Imidacloprid: pesticide tolerance for emergency exemption. Federal Registry, Washington DC, USA
- Flores CF, González PE, Fernández PM, Villafranca SM, Socías VM, et al. (2002) Effects of dissolved organic carbon on absorption and mobility of imidacloprid in soil. J Environ Qual 31(2): 880-888.
- Adedeji OB, Adeyemo OK, Agbede SA (2009) Effects of diazinon on blood parameters in the African catfish (Clarias gariepinus). African J Biotech 8(16): 3940-3946.
- Langiano VC, Martinez CB (2008) Toxicity and effects of a glyphosatebased herbicide on the Neotropical fish Prochilodus lineatus. Comp Biochem Physiol C Toxicol Pharmacol 147(2): 222-231.
- Inyang IR (2008) Haematological and biochemical responses of Clarias gariepinus to diazinon, PhD thesis. Rivers State University of science and technology, Port Harcourt, Nigeria.
- Tilak KSA, Rao DM, Devi AP, Murty AS (1981) Toxicity of carbaryl and 1-naphthol to four species of freshwater fish. Journal of Biosciences 3(4): 457-461.
- Asif A, Malik M, Chaudhry FN (2018) A Review of on environmental pollution bioindicators. Pollution 4(1): 111-118.
- Bartelme TD (2006) Metabolism, energy use and feeding behaviours in fish 5:
- APHA (American public health association) (1998) Standard methods for examination of water and wastewater APHA, Washington DC, USA.
- Ogamba EN, Izah SC Nabebe G (2015) Effects of 2, 4-Dichlorophenoxyacetic acid in the electrolytes of blood, liver and muscles of Clarias gariepinus. Nigeria Journal of Agriculture Food and Environment, 11(4): 23-27.
- Tietz NW (1976) Biuret method for the determination of total protein in serum in fundamental of chemical chemistry. WBS Saunda Co. Philadelphia, USA, pp. 310-315.
- Doumas BT, Watson WA, Biggs HG (1971) Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta 31(1): 87-96.
- Wahua TAT (1999) Applied statistics for scientific studies. Africa link books, Ibadan, Nigeria.
- Adamu KM, Kori SO (2011) Effect of sublethal concentrations of tobacco (Nicotiana tobacum) leaf dust on some biochemical parameters of hybrid catfish (Clarias gariepinus) and Heterobranchus bidorsalis. Brazilian archives of Biology and Technology 54(1): 183-193.
- Magar RS, Shaikh A (2012) Biochemical changes in proteins and amino acids in Channa punctatus in responses to sublethal treatment with the insecticide Malathion. Trend life Sci 1(3): 19-23
- Taylor DJ, Green NPO, Stout GW (2005) Biological science. Cambridge university press (3rd edn),
- Christensen GM, Tucker JH (1976). Effects of selected water toxicants on the in vitro activity of fish carbonic anhydrase. Chem Biol Interact 13(2): 181-192.
- Gabriel UU, Jack IR, Edori OS, Egobueze E (2009) Electrolytes in selected tissues of heterobranchus bidorsalis treated with sub lethal levels of cypermethrin. Ethiopian Journal of Environmental Studies and Management 2(3): 83-89.
- Ogamba EN, Inyang IR, Afor God SS (2011) Alterations in the levels of ions in muscle and liver of African catfish, Claria gariepinus exposed to praquat dichloride. Current Research Journal of Biological Science 3(6): 547-549.
- Erhunmwunse NO, Ainerua MO (2014) Effect of glyphosate herbicide (360g/l) on some biochemical electrolytes of exposed Africa catfish. IOSR Journal of Environmental Science, Toxicology and Food Technology 8(1): 27-29.
- DC Nutrition (2017) Sodium (Na).
© 2018 Sylvester C Izah. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






