- Submissions

Full Text
Environmental Analysis & Ecology Studies
Applied Studies on Material of Bio Composite Biological Origin Prepared from Oxide Nano and Bio Gum (Extracted from Osaka Seeds (Cassia Fistula Linn)) for the Treatment of Methylene Blue
Dao Minh Trung* and Truong Nguyen PV
Vietnam
*Corresponding author: Dao Minh Trung, 06 Tran Van on street, Phu hoa ward, Binh Duong Province, Thu Dau Mot City, Vietnam
Submission: June 14, 2018; Published: December 10, 2018

ISSN 2578-0336 Volume4 Issue4
Abstract
Researching on Methylene Blue dye treatment ability with Nano material (CoFe2O4 synthesized by co-precipitation method) combined with Bio gum (extracted from Osaka Seeds (Cassia Fistula Linn). Research results show that with Bio gum base material fully covered by magnetic nanoparticles of size 70-95nm (SEM) and the presence of several specific functional groups for bio gum and ferromagnetic oxide Nano (FT-IR). When adding OH group to Nano material, the adhesive ability to bio gum and the size of the particle does not change. Results of comparative studies on the application of Nano-Bio gum and Nano-OH-Bio gum materials showed that when studying the dyeing color of Methylene blue, the treatment efficiency is from 62.43 to 78.72%, decreasing from 349.67 Pt-Co. 74.41 Pt-Co at pH 6-7 and dosage at 1.5g/l. Based on the above results, Nano-Bio gum is able to treat dyeing color better than Nano OH-Bio gum. Therefore, Nano - Bio gum can become suitable material for the application of color treatment in practice.
Keywords: Nano gum; Gum OH nano; Materials of biological origin; Color treatment; Methylene blue; Treatment effiency
Overall
Dyeing textile industry is one of the chemical industries contains many pollutants discharged after several dyeing phases [1]. Wastewater from the dyeing process is discharged with relatively high color [2-4]. According to color research [2], it is capable of obstructing light and slow down the process of photosynthesis, inhibiting the growth and reproduction of organisms as well as tend to generate metal chelate ions causing toxic to bacteria in the water (Figure 1). Therefore, the direct discharge into areas such as rivers, lakes... not only affect the entire ecosystem in the water [5] but also affect the lives of the people in the neighborhood.
Figure 1:Methylene blue processing experiment layout with two types of materials, Nano - Nano bio gum and OH-bio gum.
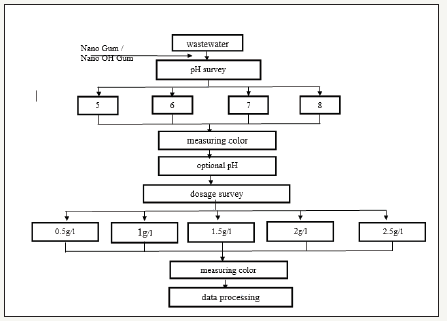
Currently, many materials of biological origin used in dyeing textile wastewater treatment such as activated carbon [6]; wheat; fly ash, zeolite clay, unburnt carbon [7,8], in addition to current Bio gum extracted from Osaka seeds (Cassia fistula Linn) is being studied and applied widely in many fields of wastewater [9], etc. materials are being used instead of the physical methods or chemical previously used because such materials are capable of completely biodegradable without generating any toxic ingredient [10,11] low cost, easy to use, easy to operate and high treatment efficiency [12].
Research on using Nano Gum and Nano OH Gum prepared from Cassia fistula seeds [13] and ferromagnetic oxide nano [14] to examine the methylene blue treatment efficiency in dyeing textile wastewater.
Research Method
Research means
Research object: Methylene Blue (C16H18CIN3S.3H2O, China) with concentration of 25mg/L.
Research chemicals: CoCl2.6H2O (China, 99%); FeCl2.4H2O (China, 98%); NaOH (China, 96%), SDS (India, 85%); Cassia fistula seeds (collected from Huynh Van Luy street, the New city, Binh Duong province); n - hexane (China, 97%); C2H5OH (China, 99.7%); NH4OH (China, 25-28%); (CH3)2CO (China, 95%), HCl (1M-China), NaOH (1M-China).
Researched materials: Nano Gum and Nano OH Gum are prepared from Cassia fistula seeds [13] and ferromagnetic oxide nano [14].
Research device: Jatest.
Research method
A. Determination of pH is measured directly using pH Meter Toledo (2017).
B. Determination of color in accordance with TCVN 6185: 2005.
C. Determination of particle size by scanning electron microscope (SEM).
D. Determination of functional groups in molecules by Fourier Transformation Infrared Spectrometer (FT-IR).
Experimental layout
Figure 2.
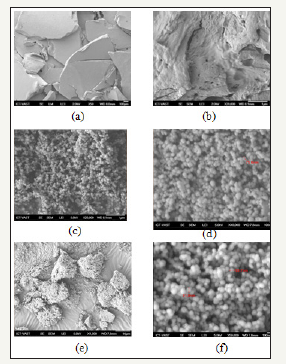
Figure 2:SEM images of the material.
Figure 2a & 2b: Bio gum material.
Figure 2c & 2d: Magnetic nano.
Figure 2e & 2f: Nano gum materials.
Results and Discussion
Material preparation results
Survey results of particle size (SEM): According to SEM results obtained in Figures 2a and 2b, MHY gum material is observed with a rough, dry surface. According to research [15], it has been shown that biogum is rSSeadily dispersible in water and has the ability to bind with water rather strongly to produce high viscosity materials that increase adhesion to contaminants. In addition, according to research results that biogum is a type of natural conglomerate, so they are able to decompose from the 2nd day at high humidity.
In Figure 2c and 2d shows ferromagnetic oxide nano is highly porous with the connection between the particles quite dense and relatively similar in size with the diameter of particles from 70- 95nm. But this size is still much larger than some other studies such as the study of [16] synthesized by the method of micro-emulsion obtained mixed particles CoFe2O4 with size of 13nm and research [17] The seed size is 15 -48nm but it is the same size with the study of [18]. According to summary from multiple studies, the size of ferromagnetic oxide nano particles depends on many factors such as pH, salt concentration, temperatures, stirring speed, concentration of SDS [19].
For Nano-Biogum material, in Figure 2e and 2f, it is shown that nanoparticle size is unchanged and highly porous clinging around Biogum bases material forming a rigid cohesive block. Because the particles bind to Biogum thickly, gravity of the particles is decreased markedly. Thereof, it shows that the solid binding ability between the nanoparticles and biogum may increase the material treatment ability.
From the infrared absorption diagram, it is seen that the presence of functional groups in the structure of Biogum as at the oscillation peaks of 3446.90cm-1 and 1636,17cm-1, these are the characteristic oscillations of the original -OH and the original -COO- [20,21]; In addition, some other oscillation peaks such as C-N fluctuations occurred at peak 1460.27cm-1 and 2925,36cm-1 characterizes C-H bond in CH2 group; there is a slight oscillation at 1409.44cm-1 is the oscillation of the two groups -OH and -CH; Group C=O was observed with slight oscillation in 1385cm-1; 1550cm-1 appeared light bonding band with amide III (group -NH3+); 1271.08 and 1040.21cm-1 oscillation correspond to the bond of C-O (CH2OH); 790, 1cm-1, it shows that this is the bond (1-4), (1-6) of galactose and mannose. Through the results of the infrared spectrum analysis FT-IR, we determined the structure of the bio-polymer extracted from osaka seeds (Cassia fistula Linn) with similar functional groups as analyzed by [20].
For CoFe2O4 particle, there was an occurrence of an oscillation peak at 3456cm-1. It is thought the oscillation peak for free-water binding and absorption on the surface of the nanoparticles (-OH group). C-H oscillation groups are observed at peak 2920cm-1 and at peak 2857cm-1 shows the presence of SDS on the surface of CoFe2O4 particle [16], 2357,55cm-1, 628,59cm-1 corresponding to the presence of -OH group. The wavelengths observed at 595.41cm- 1 and 446.44cm-1 correspond to the concave-convex vibration at the octahedral and tetrahedral compounds in the spinel structure containing Fe2+ and Co2+ groups. Compared to previous studies such as [16], there is a similarity on functional groups between the research results.
When attaching the functional groups to the surface of the nanoparticles, the oscillations at the peak of -OH group at 3456cm- 1 wavelengths is wider than those of the previous material. This could prove that nanoparticles are attached -OH-group functional groups by intermolecular hydrogen bonding. There is also a strong oscillation at the wavelengths of 595.41cm-1 and 446.44cm-1 corresponding to the internally strong oscillation of the tetrahedral structure coordinated with the octahedron in the spinel structure. It is thought that the bonding length of oxygen to metal ions (O-Me) in the octahedral holes is shorter than O-Me bonding length in the tetrahedral hole [16]. At 1628.59cm-1 wavelength, the expansive oscillation of -OH group may be due to the adsorption of H2O onto the surface of the particle.
From Figure 3, the presence of characteristic peaks of magnetic nanoparticles at 595.41cm-1 and 446.44 cm-1 wavelengths is the presence of metal bonds in tetrahedral and octahedral holes. In addition, there is the presence of -OH group characterizing for biogum at 3446,90cm-1 and 1636,17cm-1. However, Because the heated stirring process partly causes the evaporation of water molecules on the surface of the material and the breakdown of the chemical liquid bonds in the material. With the bio gum cover, the magnetism of the composite material is considerably reduced.
Figure 3:FT-IR diagram of researched materials.

According to the survey results of SEM and FT-IR image, it shows that the material has a combination of bio gum and ferromagnetic oxide Nano, it shows that the material can treat dyeing color.
Survey results of bio composite’s methylene blue color treatment
Determine optimal pH for bio composite: The research results from Figure 4 show that the color treatment efficiency is highest at pH=6 for Nano-material with - OH group attached to osaka gum and at pH 7 for Nano material. combined with Osaka gum. With the treatment efficiency of Nano gum and Nano-gum added -OH reaching at 31.42% and 30.41%. It can be explained that the influence of H+ ion on the material’s absorption through the protonation process of the functional group.
Figure 4:Color treatment efficiency of Nano Gum and Nano OH Gum material by pH.
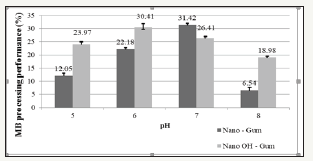
Some studies show that at low pH, the surface of Bio composite material is positively charged due to the presence of excess H+ ions and these ions compete with dye cations and reduce the absorption of Methylene Blue while at high pH, -COOH functional groups on the material surface are ionized and interact with the molecules of Methylene Blue, in addition -COOH groups contained in acrylate are separated to form -COO group which increases the ionizing groups and creates an electrostatic repulsion, which causes the polymer chains to expand and enhance the cationic absorption of the dye.
Based on several studies of other biologically derived materials such as almond gum and guar gum, it is shown pH in the range of 6 to 10 is the appropriate pH rate for color absorption of Methylene Blue with achieved efficiency of 55%.
Thus, when compared with previous studies, it is showed that there is a similarity of pH suitable for the treatment process of Methylene Blue wastewater between the materials studied. This proves that the research material is capable of treating the dyeing color.
Determining the optimal dosage for bio composite: From Figure 5, appropriate dosage survey studies show that Methylene Blue’s color treatment ability is increased from 0.5g/L to 1.5g/L, the highest concentration is at 1.5g Bio composite/L, with the efficiency of Nano OH gum reaches 62.43% and Nano gum reaches 78.72%. From the initial coloring concentration (349.67 Pt-Co) via the treatment of Nano Gum and Nano OH Gum material is reduced to 74.41 Pt-Co for Nano Gum and 131.37 Pt-Co for Nano OH Gum.
Figure 5:The color treatment efficiency of Nano Gum and Nano OH Gum by dosage
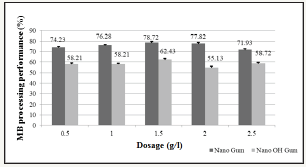
The research result on Bio composite material is compared with the previous researches shows that the efficiency of color treatment in dyeing textile wastewater is similar as the research result on the use of sludge after domestic wastewater treatment process achieved 81% or research project on Shewan Ella putrefacient cell of has eliminated 80% of color out of the solution or research results of almond gum of shows that at concentrations of 0.05-2g/L the treatment ability reaches 89%.
However, some research results such as the research results of achieved 56% eliminating efficiency when using Kurthia sp or the research of on anaerobic microorganisms with a 70% color eliminating ability, it is shown that Bio composite treatment ability is better.
Conclusion
Nano Gum and Nano OH Gum is a material of biological origin which is combined by CoFe2O4 magnetic Nano and treatment ability of Osaka gum. In this study, Nano Gum and Nano OH Gum is used to examine the color treatment ability of the initial concentration of 349.67 Pt-Co. The results show that Nano Gum is used to treat color at pH=6 and 1.5mg/L dosage reaches the efficiency of 78.72%, it is reduced from initial color concentration down to 74.41 Pt-Co.
At the same dosage, Nano material added OH-group combined with gum gave an efficiency of 62.43% at pH =7 lower than the material without functional group. Based on the research results, it shows that Nano Gum and Nano OH Gum material is capable of treating Methylene Blue in dyeing textile wastewater. The research result is the scientific basis to guide the expansion of applied research on Nano Gum and Nano OH Gum in dyeing textile wastewater treatment.
References
- Gao BY, Yue QY, Wang Y, Zhou WZ (2007) Color removal from dye containing wastewater by magnesium chloride. Journal of Environmental Management 82(2): 167-172.
- Garg VK, Amita M, Kumar R, Gupta R (2004) Basic dye (methylene blue) removal from simulated wastewater by adsorption using Indian Rosewood sawdust: A timber industry waste, Dyes and pigments 63: 243-250.
- Solmaz SK, Birgul A, Ustun GE, Yonar T (2006) Colour and COD removal from textile effluent by coagulation and advanced oxidation processes. Coloration Technology 122(2): 102-109.
- Verma AK, Dash RR, Bhunia P (2012) A review on chemical coagulation/ flocculation technologies for removal of colour from textile wastewaters. Journal of Environmental Management 93(1): 154-168.
- Wang S, Zhu ZH, Coomes A, Haghseresht F, Lu GQ (2005) The physical and surface chemical characteristics of activated carbons and the adsorption of methylene blue from wastewater. Journal of Colloid and Interface Science 284(2): 440-446.
- Bulut Y, Aydın H (2006) A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination 194(1-3): 259- 267.
- Wang S, Li L, Wu H, Zhu ZH (2005) Unburned carbon as a low-cost adsorbent for treatment of methylene blue-containing wastewater. Journal of colloid and interface science 292(2): 336-343.
- Trung DM, Hung LA, Nguyen TKT, Nguyen VCN (2016) Effectiveness on color and COD of textile wastewater removing by biological material obtained from Cassia fistula seed. J Viet Env 8(2): 121-128.
- Lee SH, Wang S (2006) Biodegradable polymers/bamboo fiber bio composite with bio-based coupling agent. Composites Part A: Applied Science and Manufacturing 37: 80-91.
- Dao MT, Nguyen VC, Ngo KD (2016) Effect of dye textile wastewater treatment of chemical and biological coagulants. Journal of Science, p. 127.
- Gao BY, Yue QY, Wang Y, Zhou WZ (2005) Color removal from dye containing wastewater by magnesium chloride. Journal of Environmental Management 82: 167-172.
- Vinod VTP, Sashidhar RB (2011) Bioremediation of industrial toxic metals with gum kondagogu (Cochlospermum gossypium): A natural carbohydrate biopolymer. pp. 113-120.
- Pui A, Gherca D, Carja G (2011) Characterization and magnetic properties of capped CoFe2O4 nanoparticles ferrite prepared in carboxymethylcellulose solution. Dig J Nanomater Bios 6: 1783-1791.
- Singh SK, Singh S (2010) Evaluation of Cassia fistula Linn. Seed mucilage in tablet formulations. International Journal of Pharm Tech Research 2(3): 1839-1846.
- Maaz K, Mumtaz A, Hasanain SK, Ceylan A (2007) Synthesis and magnetic properties of cobalt ferrite (CoFe2O4) nanoparticles prepared by wet chemical route. Journal of magnetism and magnetic materials 308(2): 289-295
- Sadri F, Ramazani A, Massoudi A, Khoobi M, Azizkhani V, et al. (2014) Magnetic CoFe2O4 nanoparticles as an efficient catalyst for the oxidation of alcohols to carbonyl compounds in the presence of oxone as an oxidant. Bullein of the Korean Chemical Society 35: 2029-2032.
- Kim YI, Kim D, Lee CS (2003) Synthesis and characterization of CoFe2O4 magnetic nanoparticles prepared by temperature - controlled coprecipitation method. Physica B 337(1-4): 42-51.
- Vinod VT, Sashidhar RB, Sukumar AA (2010) Competitive adsorption of toxic heavy metal contaminant by gum kondagogu (Cochlospermum gossypium): A natural hydrocolloid. Colloids and Surfaces B: Biointerfaces 75(2): 490-495.
- Vinod VTP, Shashidhar RB (2010) Surface morphology, chemical and structural assignment of gum Kondagogu (Cochlospermum gossypium DC.): An exudate tree gum of India. Indian Journal of Natural Products and Resources 1: 181-192.
- Andrews HE, Castilla OS, Torres HV, Carter EGV, Calleros CL (2010) Determination of the gum Arabic-chitosan interactions by fourier transform infrared spectroscopy and characterization of the microstructure and rheological features of their coacervates. Carbohydrate Polymers 79(3): 541-546.
- Mudgil D, Sheweta B, Khatkar BS (2012) X-ray diffraction, IR spectroscopy and thermal characterization of partially hydrolyzed guar gum. International Journal of Biological Macromolecules 50(4): 1035- 1039.
© 2018 Dao Minh Trung. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






