- Submissions

Full Text
Environmental Analysis & Ecology Studies
Phosphorus Speciation in the Pearl River Estuary of South China
Chengyu Chen*
College of Natural Resources and Environment, South China Agricultural University, China
*Corresponding author: Chengyu Chen, College of Natural Resources and Environment, South China Agricultural University, Wushan Road, Guangzhou, Guangdong 510642, People’s Republic of China
Submission: June 18, 2018;Published: August 16, 2018

ISSN 2578-0336 Volume3 Issue5
Abstract
Based on information of chemical properties in the water samples in the Pearl River Estuary in South China in prior studies, the speciation of phosphorus in water samples from four sampling sites was calculated and modelled by a MINEQL+4.6 Software, in an effort to quantify the likelihood and reason of eutrophication and algal bloom in the local aquatic environment. Results showed that the dominant forms of phosphorus in local water are hydroxylapatite and H2PO4-, with the minor forms being HPO4 2- and CaHPO4. The total dissolved phosphate is 4.55, 4.59, 7.06, and 7.93, 10-6mol/L for Zones A to D, respectively. Increasing Ca2+ concentration leads to precipitation and removal of phosphorus from water, but its concentration will need to be increased by 9, 10, 14.7, and 13.8 times, respectively, in Zones A to D in order to prevent eutrophication and subsequent algal blooms [1].
Introduction
Algal blooming in water environments has become a critical as well as worldwide issue for its impacts on local nature, human society, and global economics. This problem is serious in the Pearl River estuary in southern China. The Pearl River is the second longest river in China. Since the Pearl River estuary supports large population of marine organism and contributes significantly to the fisheries in South China Sea, and it is also the main receiving water of landbased pollutants of South China Sea, the water quality of the Pearl River is significant to the local natural and economic environment [2]. The massive economic developments in recent decades in China have caused excessive inputs of nutrients such as nitrogen and phosphorus into the aquatic system in the Pearl River. This process, known as eutrophication, leads to algal bloom that dramatically reduces the dissolved oxygen (DO) concentration and causes fish death in the aquatic environment.
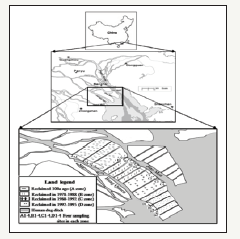
This paper focuses on the chemical speciation of phosphorus (P) in a study area located in the north of the Pearl River estuary, Guangdong Province, South China (22°36’39’’-22°44’36’’N and 113°23’42’’-113°38’34’’E) [1]. It covers approximately 2250hm2 and has a subtropical maritime monsoon with annual mean temperature of 23.2 °C and mean annual precipitation of 1655.7mm. Four zones in the Pearl River estuary, named A, B, C and D, with different reclamation histories, 1978-1988, 1988-1992, and 1992- 1995, respectively, were examined in this study to compare the difference in their phosphorus concentration (Figure 1).
Phosphorus mainly exists in the form of phosphate (PO4 3-), with a common phosphate concentration in the Pearl River estuary be ing around 0.015mg/L [2]. However, the studied system contains various phosphate concentrations that were quite above this common phosphate concentration, which may cause eutrophication. Sources of phosphate in the estuary regions include runoff of river, estuarine land-based discharge, and coastal land-based pollutants coming with South China coastal current and atmospheric deposition. The main source of phosphate is the land-based sources such as the leaking of fertilizer from estuary being.
Figure 1:Locations of the sampling sites in the fringe marshes of the Pearl River Estuary Xiao et al. [1].
Phosphorus does not undergo oxidation-reduction in the aquatic system, but it forms precipitation with other components in the aquatic system. Therefore, the sink of phosphate mainly results from its precipitation into sediments of the Pearl River estuary. The three types of precipitates-hydroxylapatite (Ca10(PO4)*6(OH)2), aluminium phosphates (AlPO4), and strengite (FePO4)- are the major forms that remove phosphorus from the aquatic system [1]. These precipitates are formed during reaction of phosphate with Ca2+, Fe3+, and Al3+, which constitutes the main sinks of phosphate.
Figure 2:Calculated phosphate speciation in the four zones.

Aim of Study
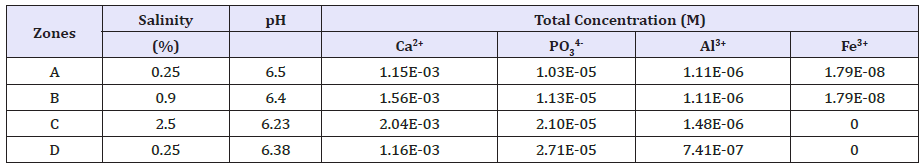
Four research zones (A-D) in the Pearl River estuary (Figure 1) with different total Ca2+, Fe3+, Al3+, and PO4 3- concentrations due to different reclamation histories were chosen for calculation. Basic water conditions, including salinity, pH, and ionic concentrations for Ca2+, PO3 4-, Al3+, and Fe3+ are reported by previous study (Table 1).
Table 1:Water properties in the overlying water of the four sampling sites. Rearranged from Xiao et al. [1].
Based on the known information, this paper aims to calculate the speciation of phosphorus in the Pearl River of southern China, in an effort to assess the contribution of phosphorus to eutrophication and to identify the role of precipitation in removal of excessive phosphorus from water. The contribution of each precipitate, such as hydroxylapatite (Ca10(PO4)*6(OH)2), aluminium phosphates (AlPO4), and strengite (FePO4) to removal of phosphate at the local pH and ionic concentrations from water will be quantified. The free phosphate concentrations remaining against the addiction of the chemical that is most effective in precipitating phosphate will be quantified using the MINEQL+4.6 Software. The dosage of chemicals added to each system for precipitation and removal of phosphate to below 20μg/L will be calculated.
Phosphorus Precipitation Reactions
Three main multivalent metal ions precipitate phosphate in natural aquatic systems and also in wastewater treatment processes: calcium, aluminum, and iron. The excessive calcium ions after reacting with natural alkalinity will then react with phosphate to precipitate in the form of hydroxylapatite (Equation 1). The stability constant for this reaction, Log K1, is -44.333.
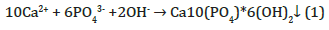
Alum or hydrated aluminum sulfate can also precipitate phosphate to form aluminium phosphate (AlPO4) (Equation 2). This process depends on the competing reactions, equilibrium constants alkalinity, pH, and trace metals, with a stability constant Log K2 of -18.2.
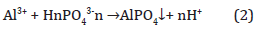
Ferric ions also react with phosphate to form precipitate strengite (Equation 3), with a stability constant Log K3 of -26.4. However, since iron is a limiting nutrient for aquatic environments, its addition to aquatic system will promote algal bloom.
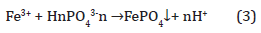
Result and Discussion
The phosphate speciation calculated based on the total Ca2+, Al3+, Fe3+, and P concentrations in each zone are shown in Figure 2. It shows that the dominant forms of phosphate in these zones are hydroxylapatite and H2PO4-, with the minor forms being HPO4 2- and CaHPO4. Since only the dissolved form of P contributes to eutrophication and the major precipitates are hydroxylapatite, which forms by reaction between calcium and phosphate, Ca2+ should be the control factor of the phosphate speciation.
Figure 3 shows that the total dissolved phosphate is 4.55, 4.59, 7.06, and 7.93, 10-6mol/L (M) for Zone A, B, C, and D, respectively. These molar concentrations were converted into mass concentrations, giving total phosphorus (TP) cocentrations of 141, 142, 219, and 246μg/L for Zones A to D, respectively. This suggests that all four zones are at eutrophic state and algal bloom events are likely to happen. Zone D has the highest TP concentration among the four zones likely because it has the highest P concentration as well as very low Ca2+ concentration (Table 1).
Figure 3:Changes in phosphorus speciation due to the increase in Ca2+ concentrations from current TP concentration to meet TP requirement for 20μg/L (i.e. 2.10E-7M) in all zones.
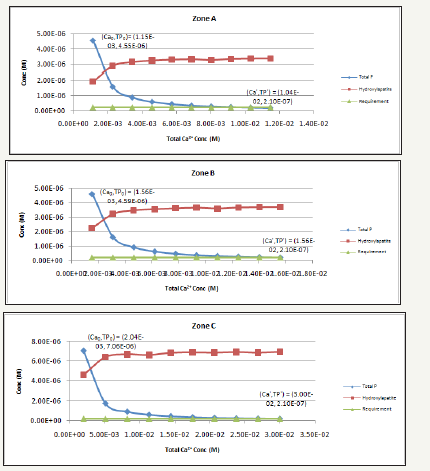
Figure 3 also indicates that as Ca2+ concentration increases, the TP concentrations in all four zones decrease rapidly and graduately reach 20μg/L (i.e. 2.10E-7M) by precipitating phosphate in the form of hydroxylapatite (Ca10(PO4)*6(OH)2). In order to precipitate the dissolved phosphorus and achieve its required concentration of 20μg/L, the Ca2+ concentrations will need to be increased by 9, 10, 14.7, and 13.8 times, respectively, from its current concentration in Table 1.
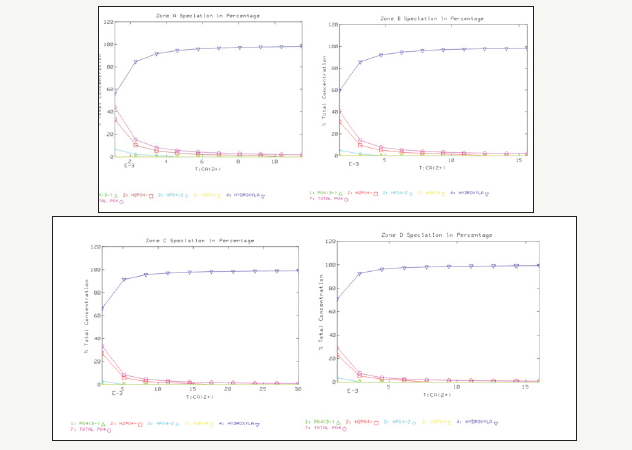
Figure 4 further demonstrates the effect of increasing Ca2+ concentrations on the speciation of phosphate. The increase of calcium concentration converts the dissolved phosphate into hydroxylapatite (Ca10(PO4)*6(OH)2) precipitate, which lowers the TP concentration below 20μg/L (i.e. 2.10E-7 M) for all four zones.
Conclusion
Based on the MINEQL results, the dominant forms of phosphate are hydroxylapatite and H2PO4- and the minor forms are HPO42- and CaHPO4. Increasing Ca2+ concentration leads to precipitation and removal of phosphorus from water, which may prevent the occurrence of eutrophication and subsequent algal blooms.
Figure 4:Effect of increasing calcium concentration on phosphate speciation in the four zones.
References
- Xiao RJ, Bai H, Zhang H, Gao X, Liu A, Wilkes (2011) Changes of P, Ca, Al and Fecontents in fringe marses along a pedognic chronosequence in the Pearl River estuary, South China, Continental Shelf Research 31: 739-747.
- Huang XP, Huang LM, Yue WZ (2003) The characteristics of nutrients and eutrophication inthe Pearl River estuary, South China. Marine Pollution Bulletin 47(1-6): 30-36.
© 2018 Chengyu Chen. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






