- Submissions
Full Text
Environmental Analysis & Ecology Studies
Radiological Impacts of Norm and Poly Aromatic Hydrocarbon in Petroleum Industry Process on Marine Ecosystem at the Red Sea, Egypt
Kh M Zakaria*
Nuclear and Radiological Regulatory Authority, Egypt
*Corresponding author: Kh M Zakaria, Nuclear and Radiological Regulatory Authority, Nasr City, P O Box 7551, Cairo, Egypt
Submission: January 25, 2018; Published: February 27, 2018

ISSN: 2578-0336 Volume1 Issue4
Abstract
This study was designed to realized the hazardous effects on marine ecosystem and assess the impacts of (NORM) 238U, 232Th, and 40K radioactivity and Polycyclic aromatic hydrocarbons in petroleum industry at red sea coastline in Abu Zenima and Abu Rudeis sites. Polycyclic aromatic hydrocarbons(PAHs) individuals Naphthalene, Acenaphthalene, Acenaphthylene, Fluorene, phenanthrene and Fluoranthene were analysed with HPLC with fluorescence detection for sea water, sediments and marine organisms Bivalves, coral reef and starfish. HPGe gamma ray spectrometric technique used for measured the 238U, 232Th, and 40K radioactivity concentration in sea water, sediments and marine organisms Bivalves, coral reef and starfish in both Abu Zenima and Abu Rude is sites. The data revealed an increase in the 238U, 232Th, and 40K and (PAHs) individuals for sea water, sediments and marine organisms Bivalves, coral reef and starfish in Abu Zenima and Abu Rude is sites respectively as compared to international maximum allowable concentrations. On the other hand, 238U, 232Th, and 40K and (PAHs) individuals for sea water, sediments and marine organisms Bivalves, coral reef and starfish were recorded higher concentration levels in Abu Zenima sitesthan Abu Rudeis sites. The data revealed an increase in concentration of (NORM) 238U, 232Th and 40K and PAHs individuals for starfish and coral reef than bivalves marine organisms for both Abu Zenima and Abu Rudeis sites respectively as compared to international maximum allowable concentrations.
Keywords: NORM; Poly aromatic hydrocarbon; Petroleum industry; Radiological impacts; Marine ecosystem
Introduction
The oil and hence polycyclic aromatic hydrocarbons (PAHs) are naturally present in the marine environment, although levels have increased significantly following human extraction and use of oil and gas [1]. Naturally occurring radioactive materials (NORM) generally contain radionuclides found in nature, i.e., thorium, uranium, and their progeny [2]. The TENORM waste is produced from several industrial sectors such as uranium and metal mining, phosphate ores? Processing, and petroleum industry [3]. The petroleum waste (scale or sludge) have been produced by two mechanisms: either incorporation or precipitation onto the production equipment such as: pipelines, storage tank, pumps [4]. The precipitated TENORM wastes around walls of the petroleum pipes reduce their efficiency and then disposed and replaced periodically by new ones [5]. In the last decade, attention was focused on the environmental and health impact from the release of TENORM wastes.
Studies of naturally occurring radioisotopes of uranium and thorium decay series and primordial potassium in aquatic environment provide information on the environmental pollutants in water bodies, whereas marine invertebrates include sea slugs, sea anemones, starfish, octopuses, clams, sponges, sea worms, crabs, lobsters, coral reef and Bivalves, most of these animals are found close to the shore [6]. The possible radionuclide transfer provide an easy assimilation of radiation exposure into human body following their consumption [7,8]. Moreover, marine organisms such as filter feeders and piscivorous have the capacity of bio accumulating more radionuclides and toxic elements from water due to the physical and chemical nature of their body surfaces and feeding habit in their natural habitat, and thus, the determination of radioactivity in the marine organisms and estuaries marine animals assumes greater importance [8]. The level of natural radio nuclides in marine organisms (226Ra, 228Ra and 40K) in mostly consumed marine fishes and shell fishes due to their importance as sources of high-quality protein [9]. Releases of radionuclides TENORM from marine petroleum industry (226Ra, 228Ra and 40K) effects on aquatic animals and marine food chains [9]. Among the many radionuclides discharged into marine environments, (226Ra, 228Ra and 40K) the fate of these radionuclides in the aquatic food chain is essential for a realistic assessment of the risk of their potential impact on human health [10]. The contribution of naturally occurring radionuclides reflected in the sediments and marine coastline water. The radiation dose received and accumulated in the body by marine fauna comes from the naturally occurring uranium series [11].
On the other hand petroleum industry in marine have the polyaromatic hydrocarbons (PAHs) beside 238U, 226Ra, 232Th and 40K are formed during the manufactured oils in marine and sea water [11]. Oil and its derivatives PAHs have been found to be toxic to a wide array of marine organisms from marine invertebrates to large seabirds and marine mammals [12]. The effects of PAH son single organisms may add up to affect populations and communities, as well as whole ecosystems [12]. Phytoplankton, the major primary producer in the marine environment, is also sensitive to environmental stressors such as pollution. Phytoplankton plays a crucial role in the inflow of primary energy to coastal food webs, thereby changes in phytoplankton may cause significant damages to the functioning of marine ecosystems [13]. Abu Zenima and Abu Rudeis located on Suez gulf, this area have many oil offshore platform, several of these offshore platforms have been in operation to extract and process oil, processing and petroleum products distribution activities and have resulted in tremendous impacts on the safety and environmental conditions in the Suez Gulf. PAHs leaks and spills have resulted in serious contamination to the Suez gulf. PAHs contain many aromatic generation compounds such as naphthalene, acenaphthalene, acenaphthylene, fluorene, phenanthrene, fluoranthene. Each PAHs can also be released into the environment, mainly due to offshore oil production or petroleum transportation [14]. Each PAHs have bad impacts contamination on bivalves and particularly mussels coral reef and star fish and marine invertebrates the status of the marine environment for large number of pollutants [15]. Where, PAHs are lipophilic and coplanar; they can accumulate in adipose tissues or secretions. Coral reefs are threatened by small chronic PAHs spills in particular, but larger acute oil spills may also affect coral reefs [15]. Observed biological impacts of oil spills in reef areas range from mass mortality of fish and invertebrates to apparently marine ecosystem devastation [16]. PAHs components can dissolve in water to some extent which exposes the corals to potentially toxic compounds. However, toxic concentrations are only encountered in the uppermost part of the water-column [16]. In the Suez Gulf PAHs easily gets trapped in the mangroves and usually persists for a very long time. The PAHs is subject to microbial degradation which may be a rather rapid process in aerobic environments. However, if the PAHs are buried within the anaerobic sediments, bio-degradation proceeds very slowly [17].
Materials and Methods
Study area
The investigated area expanded along the coast of Suez gulf, Abu Zenima 29°3’20"N 33°6’U"E to Abu Rudeis 28°53'N 33°11'E.
Sampling and sample preparation
Six representative superficial shore sediment, sea water and marine organisms samples (Bivalves, Coral reef and Star fish) were collected from six different stations located along the offshore oil platforms in Abu Zenima (Abu Zenima 1, Abu Zenima 2 and Abu Zenima 3), and Abu Rudeis 1, Abu Rudeis 2 and Abu Rudeis 3). An area of about 25x25cm2 up to a depth of 5cm was cut out using the stainless steel template for guidance [18]. The collected shore sediment and sea water samples were transferred to labelled polyethylene bags, closed and transported to the laboratory for preparation and chemical analysis. The shore sediment samples were air-dried at room temperature for a week. And also were dried in an oven at 80 °C (for 48h) till constant dry weight was obtained, crushed and homogenized. Then milled and sieved through 0.4mm mesh sieves and stored for further analysis. Water samples, 5 liter of each, were collected using the water sampler. They were collected in polyethylene containers. Then, the samples were acidified with Nitric acid to pH lower than 2 to avoid microorganisms growth. The samples were stored for radioactivity and Polycyclic aromatic hydrocarbons measurements. The marine organisms samples of Coral reef, Bivalves and Star fish were collected from Abu Zenima and Abu Rudeis and transported to the laboratory in ice boxes and stored at (-10 °C) until subjected for further analysis, about 20 individuals of each species were collected from the study area; at the same locations of the water samples. The samples of marine organisms were then cut into smaller pieces to ensure effective grinding. The cleaned samples were dried in an oven at 70 °C for five days (until there was no detectable change in the mass of the samples) to ensure that the sample were completely moisture free, a constant dry weight being obtained. Dried samples were ground to fine grain sizes by using a stainless steel cutter blender, and sieved in order to obtain homogeneity. All homogenized samples were divided into two parts. The first part was transferred into 250ml sizes Marinelli beaker, sealed hermetically, and left for about 4 weeks at room temperature in order to attain secular equilibrium among the 238U series and 232Th series precursors with their short-lived progenies [19]. The second part was kept in laboratory room temperature for using it in analysis the polycyclic aromatic hydrocarbons Individuals.
Radioactivity measurements
The activity concentration of the natural radioactivity 226Ra, 228Ra and 40K in the investigated samples were determined using a high-resolution HPGe y-spectrometry system with 30% counting efficiency. This was performed by taking 250cm3 counting vials filled up to a height of 7cm, which correspond to 170cm3. The measurement duration was up to 80000sec and was carried out in the Laboratory of Egyptian Nuclear and Radiological Regulatory Authority. The obtained spectra were analyzed. The determination of the presence of radionuclides and calculation of their activities were based on the following gamma-ray transitions (in keV): the 226Ra activities (or 238U activities for samples assumed to be in radioactive equilibrium) were estimated from 234Th (92.38keV, 5.6%), while y-energies of 214Pb (351.9keV, 35.8%) and 214Bi (609.3, 45%), 1764.5keV, 17%) and 226Ra (185.99Ke V, 3.5%) were used to estimate the concentration of 226Ra. The Gamma- ray energies of 212Pb (238.6keV, 45%), and 228Ac (338.4keV, 12.3%), (911.07keV, 29%), (968.90keV, 17%) were used to estimate the concentration of 232Th. The activity concentrations of 40K were measured directly by its own gamma rays (1460.8keV, 10.7%). In order to determine the background distribution due to naturally occurring radionuclides in the environment around the detector, an empty polystyrene container was counted in the same manner as the samples. The activity concentrations were calculated after measurement and subtraction of the background. The activities were determined from measuring their respective decay daughters [20,21].
PAHs (Polycyclic aromatic hydrocarbons) measurements
Water samples (2.5L) were collected in glass bottles at the water surface and 50cm below water level from the six different sites on Abu Zenima and Abu Rudeis. The bottles were covered with screw caps and the samples were immediately transported to the laboratory for analysis. Water samples were filtered to remove sand and debris. Sediment samples (about 2kg for each sample) were taken from the same locations and time for water sampling at a depth 5cm of sediment surface. The water was removed from the sediments by decantation and then transferred to the laboratory. Samples were air dried in dark for 48 hours before analysis. Marine organisms (Bivalves, coral reef and star fish) were caught by fishermen three representative samples from the different sites at Abu Zenima and Abu Rudeis at the same times as water and sediment sampling. They were transport to the laboratory. Standard solutions of reference materials were prepared in hexane. A stock solution containing the following PAHs was used for quantitation: naphthalene, acenaphthalene, acenaphthylene, fluorene, phenanthrene, fluoranthene by dilution to create a series of calibration standards of PAHs at 0.1, 0.25, 0.5, 1.0, 5.0 and 10|ig/ml. All solvents used in this study were of HPLC grade and were purchased from Alliance Bio, USA. Anhydrous Sodium sulfate and potassium hydroxide were of analytical grade PAHs were analyzed by using HPLC with fluorescence detection at Chemistry Department, Egyptian Petroleum Research Institution (EPRI). The mobile phase was a mixture of acetonitrile and water with a gradient concentration mode of acetonitrile. The flow rate was 1mL/min. The time program of the fluorescence detector was set to detect at optimum excitation and emission wavelength for each PAHs according to laboratory detection method [22,23].
Results and Discussion
Radioactivity and radionuclide in water and sediment in coastline in Abu Zenima and Abu Rudeis
Table 1: Concentration of 226Ra, 228Ra and 40K in seawater and coastal sediments collected from different locations along the Abu Zenima coastline.
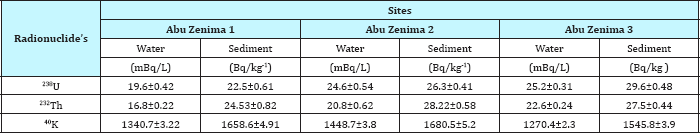
Table 2: Concentration of 226Ra, 228Ra and 40K in seawater and coastal sediments collected from different locations along the Abu Rudeis coastline.
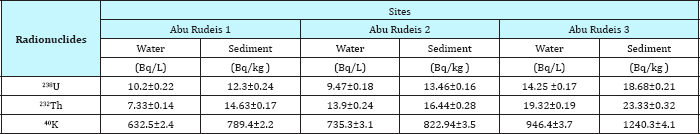
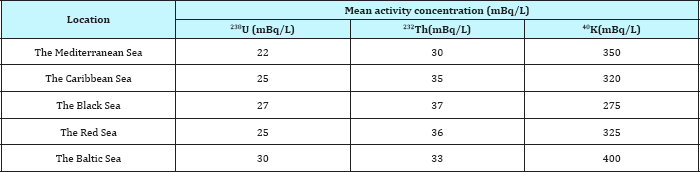
Table 1 reveled the activity concentrations of, 238U, 232Th and 40K for sea water coastline in three sites at Abu Zenima1, Abu Zenima2 and Abu Zenima3.The activity concentrations of 238U, 232Th and 40K in seawater at site of Abu Zenima1 19.6mBq/L, 16.8mBq/L and 1340.7mBq/L respectively. On the other hand 238U, 232Th and 40K recorded in Abu Zenima 2 highly activity 24.6mBq/L, 20.8mBq/L and 1448.7mBq/L respectively. Also in site of Abu Zenima3238U, 232Th and 40K were recorded 25.2mBq/L, 22.6mBq/L and 1270.4mBq/L respectively. On the other hand Table 2 reveled 238U, 232Th and 40K in sea water at Abu Rude is 1 recorded 10.2mBq/L, 7.33mBq/L and 632.5mBq/L respectively. Also 238U, 232Th and 40K recorded in sea water at Abu Rude is 29.47mBq/L 13.9mBq/L and 735.3mBq/L respectively. Also Table 3 revealed levels of 238U, 232Th and 40K in sea water at Abu Rudeis (3)14.25mBq/L, 19.3mBq/L and 946.4mBq/L. Regarding to the present data and in compared to International permissible limits for NORMS radionuclide's in Table 4, where data in the present study for 238U, and 232Th lay under the International permissible limits for NORMS radionuclide's in red sea 25mBq/L and 3mBq/L for 238U, and 232Th respectively. On the other hand El Afifi et al. [24] was recorded high levels activity concentration for 238U, 232Th and 40K in the TE-NORM waste at four different sites for petroleum and gas production in Egypt, in the South Sinai Governorate, in the Suez Gulf area, in the Matrouh Governorate, Abu Rudeis onshore oil and gas field and Gabal El Zeit offshore oil and gas field at the Suez Gulf. The results showed that the average activity concentrations of 226Ra changed between 5.9 and 68.9kBq/kg (dry weight) in the waste samples from Gabal El Zeit and Abu Rudeis [4] fields, respectively. In Gabal El Zeit field, the lower activity concentrations (28.6kBq/kg) of 226R were found in granular sample, while higher values (56.6kBq/kg) were found in the massive samples [25,26].
Table 3: Concentration of 238U, 232Th and 40K in marine organisms collected from different locations along the Abu Zenima coastline.
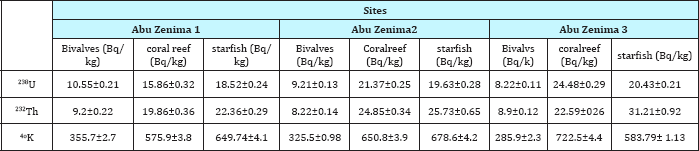
Table 4: International permissible limits for NORMS radionuclides 238U, 232Th and 40K in sea water mBq/L (EPA, 1997).
Regarding the Table 1 reveled sediment concentration of 238U, 232Th and 40K for sea water coastline at Abu Zenima 1, Abu Zenima 2 and Abu Zenima 3, where 238U, 232Th and 40K recorded in sediment coastline at Abu Zenima 1 22.5Bq/kg-1, 24.53Bq/kg-1 and 1658.6Bq/kg-1 respectively. On the other hand Abu Zenima 2 recorded highly concentration in where 238U, 232Th and 40K in costal sediment than Abu Zenima 1, where concentrations levels 26.3Bq/kg-1, 28.22Bq/kg-1 and 1680.5Bq/kg-1 respectively. Also Abu Zenima 3 recorded moderate levels for 238U, 232Th and 40K in costal sediment. Also Table 5 reveled sediment concentration 238U, 232Th and 40K for sea water coastline at Abu Rudeis (1), (2) and (3) sites. The Abu Rudies (3) site recorded highly concentration 238U, 232Th and 40K than Abu Rudeis (1), (2), at levels 18.68Bq/kg- 1, 23.3Bq/kg-1 and 1240.3Bq/kg-1 respectively. This results were confirmed with Uosif et al. [27], who noticed highly activity in 232Th and 40K in beach sediment at Suez gulf. On the other hand Table 4 highly activity in 232Th and 40K in beach sediment at Suez gulf as compared to North east coast of Tamilnadu, India and other studies in different beaches of the world. The Egyptian beach sediments recorded 238U, 232Th and 40K 26.53Bq/kg-1, 177Bq/kg-1 and 815Bq/kg-1 respectively. Many causes for increased the level of concentration 238U, 232Th and 40K in Red sea coastline [28]. The petroleum industries in red sea coastline are considered the highly important factors for increased activity concentration in Red sea coastline, the scales and petroleum sludge contains many radioactive nuclides and highly concentration NORMS and TE-NORMS. By the way many companies in past time following the regulations and criteria for radiation safety regarding waste of NORMS and TE-NORMS. These regulation which settled by and ICRP and IEAE. After year of 2012 most of petroleum companies have many problems in cost for preservation of radioactive waste. The decreased price of petroleum oil effect on regulation issues especially environmental safety and eco-marine safety. So most of the petroleum companies in Red sea coastline discharge the sludge and oil waste in sea water. Also many petroleum accident take place in these industries, which resulting of discharged the huge of petroleum oils in sea water. On the other hand, cracks in petroleum pipelines under sea water led to discharged oils and formed oils spots in coastline of sea water. Gazineu & Hazin [29], were recorded average activity concentration of 238U, 232Th and 40K in the scale, sludge and sand samples collected from disposal wasted petroleum pipes concentration of soil (33 and 45Bqkg-1 for 238U and 232Th series respectively) which indicates its contamination with radionuclide from TENORM in the wasted petroleum pipes. 228Ra activity concentration is ranged from 32 to 50kBqkg-1 for Scale and from 1 to 1.9kBqkg-1 for sludge. The sand samples have average 228Ra activity concentration equal to 0.042kBqkg-1 which within the worldwide average [30]. Many oil-field brines are particularly rich in chloride, enhancing the solubility of other elements including the radioactive element radium. Radium concentrations tend to be higher in more saline water [31]. Some of this saline, radium bearing water is also extracted with the oil and gas. Some radium and radium daughter compounds are slightly soluble in water and may become mobilized when this production water is brought to the surface [32].
Radioactivity and radionuclide for marine organisms in coastline at Abu Zenima and Abu Rudeis sites
Table 5: Comparison of activity concentrations of 238U, 232Th and 40K in beach sediment samples of North east coast of Tamilnadu, India and other studies in different beaches of the world.
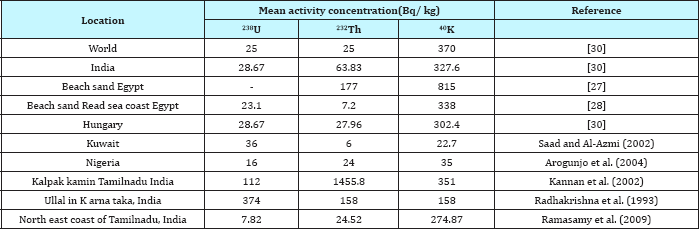
Table 5 reveled the 238U, 232Th and 40K concentration in marine organisms Bivalves, coral reef and star fish at red sea coastline in Abu Zenima sites. The concentration of 238U, 232Th and 40K in Bivalves in Abu Zenima 1 10.55Bq/kg, 9.2Bq/kg and 355.7Bq/ kg respectively. Also the coral reef recorded in Abu Zenima 1 15.86Bq/kg, 19.86 and 575Bq/kg respectively. The star fish was recorded 18.52Bq/kg, 22.36 and 649.7 in Abu Zenima 1. On the other hand Table 5 reveled the highly concentration for 238U, 232Th and 40K in coral reef and star fish at Abu Zenima 2 and Abu Zenima 3, where coral reef recorded in Abu Zenima2 21.3Bq/kg, 24.85Bq/ kg and 650.8 respectively, Also coral reef in Abu zenima3 recorded 24.48Bq/kg, 22.59Bq/kg and 722.5Bq/kg respectively. On the other hand Table 6 reveled marine organisms Bivalves, coral reef Table 6: Concentration of 238U, 232Th and 40K in marine organisms and star fish Abu Rudeis sites recorded low concentration activity in238U, 232Th and 40K than Abu Zenima sites. The coral reef and star fish was recorded highly activity concentration than Bivalves in238U, 232Th and 40Kwhere coral reef in Abu Rudeis1 11.34Bq/kg, 12.61Bq/kg and 346.81Bq/kg respectively, and in Abu Rudeis2 11.37Bq/kg, 13.85Bq/kg and 396.83Bq/kg respectively, while more activity concentration for 238U, 232Th and 40K in coral reef was recorded in Abu Rudeis3 12.22Bq/kg, 15.49Bq/kg and 466.6Bq/ kg respectively. On the other star fish recorded highly activity concentrations in 238U, 232Th and 40K at Abu Rudeis3 than Abu Rudeis1 and Abu Rudeis2, 19.82Bq/kg, 22.21Bq/kg and 520.4Bq/ kg respectively.
Table 6: Concentration of 238U, 232Th and 40K in marine organisms collected from different locations along the Abu Rudeis coastline.
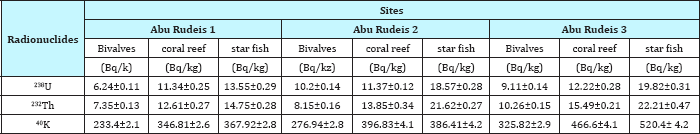
Table 7: International permissible limits for238U, 232Th and 40K (Bq/kg dry weight) in some marine biota in sea water, IEAE (1990), US EPA (1999).
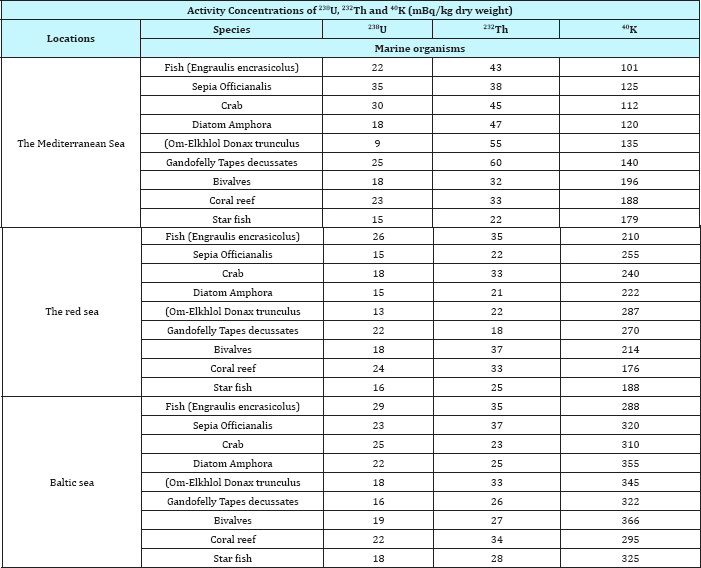
Regarding to our results and compared to International permissible limits for 238U, 232Th and 40K (Bq/kg dry weight) in some marine biota in sea water. Our results confirmed with IEAE [33] and US EPA [34] in (Table 7) where reveled the activity concentration in red sea of 238U, 232Th and 40K for many biota and marine organisms like Fish (Engraulis encrasicolus), Sepia Officianalis, Crabs, Diatom Amphora, (Om-Elkhlol "Donax trunculus, Gandofelly" Tapes decussates, Bivalves, Coral reef and Star fish. Bivalves was recorded activity concentration for 238U, 232Th and 40K 18Bq/kg, 37Bq/kg and 214Bq/kg respectively. Also coral reef and star fish in red sea were recorded 24Bq/kg, 33Bq/ kg, 176Bq/kg, 16Bq/kg, 25Bq/kg and 188Bq/kg respectively When compared the activity concentration of 40K in marine biota Bivalves, Coral reef and Star fish at red sea coastline in Abu Zenima and Abu Rudeis sites, the highly activity and increased concentration of 40K may be due to the petroleum industry in marine and petroleum platforms. This platforms discharged huge amount of petroleum waste in red sea coastline. On the other hand our results in Table 4 & 6 reveled the marine organisms have different concentration in 238U, 232Th and 40K. The Bivalves recorded low concentration for 238U, 232Th and 40K, but coral reef and star fish recorded highly concentration for 238U, 232Th and 40K in Abu Zenima and Abu Rudeis sites. The morphological structures and also chemical structures of Bivalves, where Bivalves have smooth surfaces from two sides and have no pores and have no pits on exoskeleton of their body. The morphological structures gained it more resistance against accumulation of oils waste, also the smooth exoskeleton prevents discharged the waste into internal organs for organism.
Table 8: Distribution and concentration levels of poly aromatic hydrocarbons PAHs found in seawater and coastal sediments in Abu Zenima coastline.
On the other hand coral reef and star fish have roughly surface and contain more small pores on exoskeleton. Also the coral reef and star fish contain many pits and spiny exoskeleton. Morphological structures for coral reef and star fish led to make preservation ofthese organisms with huge amount form petroleum oil waste. So the elimination process from oil waste in coral reef and star fish become more difficult than any other types of marine organisms. By the way elimination and biodegradation process of 238U, 232Th and 40K and other radioactive waste become very slowly and take more time. This data conformed with Khan[35], who study the distribution of natural radionuclides like 238U, 232Th and 40K 238U and man-made radionuclides in the dietary sources such as fish, crab, prawn, salt and drinking water from the aquatic environment. The natural radionuclides activity concentrations of in238U, 232Th and 40K 14.9, 22, 64Bqkg-1 in marine samples fishes, Crab and Prawn. Among different type of marine fishes analysed, the maximum 226Ra activity of 22Bqkg-1 was observed in the fish Sardinella sp. and Liognathus sp. was found to accumulate the highest 228Ra activity level [36]. Among the shells of animals tested, Crustacean exoskeleton registered relatively higher level of 238U, 232Th and 40K as compared to the shell of molluscan species. This is attributed to its chemical nature [37].
Poly aromatic hydrocarbons PAHs for seawater and coastal sediments at Abu Zenima and Abu Rudeis
Table 9: Distribution and concentration levels of poly aromatic hydrocarbons PAHs found in seawater and coastal sediments in Abu Rudeis
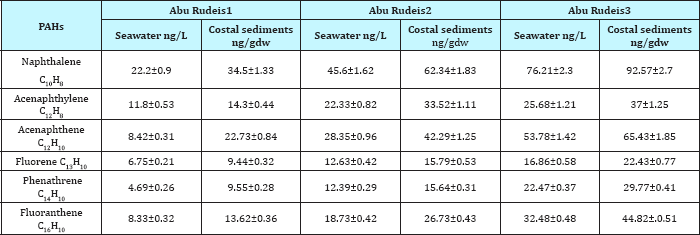
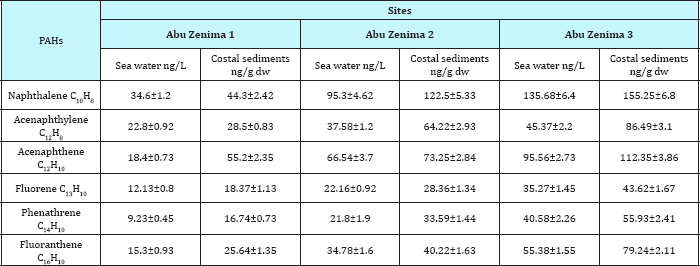
Polycyclic aromatic hydrocarbons (PAHs) are known as prevalent contaminants in marine environment. Table 8 & 9 showed the distribution and concentration levels of Poly aromatic hydrocarbons PAHs found in seawater and coastal sediments in Abu Zenima and Abu Rudeis coastline. Our results revealed that increased concentration levels of PAHs in Abu Zenima sites than Abu Rudeis sites. The data recorded that Naphthalene, Acenaphthylene, Acenaphthene, Fluorene, Phenathrene and Fluoranthene in sea water at Abu Zenima1 34.6, 22.8, 18.4, 12.13, 9.23 and 15.3ng/L respectively. On the other hand Abu Zenima 2 and Abu Zenima 3 recorded highly levels for PAHs, Naphthalene, Acenaphthylene, Acenaphthene, Fluorene, Phenathrene and Fluoranthene 95.3, 37.58, 66.54, 22.16, 21.8 and 34.78ng/L in Abu Zenima 2 respectively; 135.68,45.37, 95.56, 35.27, 40.58, 55.38ng/L in Abu Zenima 3 respectively. On the other hand and regarding to results in Table 9 showed concentration levels of Poly Aromatic Hydrocarbons PAHs found in seawater at Abu Rudeis. The Naphthalene, Acenaphthylene, Acenaphthene, Fluorene, Phenathrene and Fluoranthene in Abu Rudeis 1 were recorded levels 22.2, 11.8, 8.42, 6.75, 4.69, 8.33ng/L respectively. On the other hand Table 9 showed highly concentration levels of PAHs in seawater at Abu Rudeis2 and Abu Rudeis3 were Naphthalene, Acenaphthylene, Acenaphthene, Fluorene, Phenathrene and Fluoranthene recorded in Abu Rudeis2 45.6, 22.33, 28.35, 12.63, 12.39 and 18.73ng/L respectively; also Abu Rudeis 3 were recorded 76.21, 25.68, 53.78, 16.86, 22.47and 32.48 respectively. Spilled petroleum products are the largest single source of PAHs [38].
Table 10: Distribution and concentration levels of PAHs found in marine organisms of different sampling sites in Abu Zenima coastline.
Crude oils contain up to 10 percent PAHs, while the PAH content of shale oils and coal-derived synthetics can be as high as 15 percent. Incomplete combustion of wood and fossil fuels are important sources, as are incineration of garbage, steel and coke production, coal liquefaction, and coal gasification. Although most emissions stem from human activities, there are some natural sources such as microorganisms that are known to produce small amounts of PAHs. Produced water from both oil and gas platforms contains PAHs. Taking into account the large volumes of produced water discharged from oil production, the yearly input of PAHs into the environment, even from a single offshore oil field, may be significant. These results was found to be in agreement with that observed by Omayma [39], who observed that the petroleum company's takes water from Suez bay and mixed it with fresh water to utilize in washing the crude oil and different cooling purposes, some oily smuggling occurs for water. The oily water is characterized by that they contain a high proportion of the oils which is caused by refinery production units (Coking-distillation- oils), liquidation warehouses crude and petroleum products, water waste companies neighboring filter wards pumps, Trenchant grids crude and petroleum products. The increased of PAHs was due to found the more petroleum operations in this site. On the other hand the treatment plant cannot process in the company anymore, which is directly (untreated) discharged into Abu Zenima sites. Also the conjugated crude and petroleum products, which have TE-NORM and PAHs may be increased for marine environmental pollutants stress.
The sediments and biota, Bivalves, coral reef and starfish were affected by different concentration of PAHs. Table 8 & 9 showed an increase in PAHs Naphthalene, Acenaphthylene, Acenaphthene, Fluorene, Phenathrene and Fluoranthene at Abu Zenima sites than Abu Rudeis sites. The sediments in Abu Zenima 1 were recorded for PAHs Naphthalene, Acenaphthylene, Acenaphthene, Fluorene, Phenathrene and Fluoranthene 44.3, 28.5, 55.2, 18.3, 16.74 and 25.64ng/gdw respectively as compared to Maximum allowable concentrations (Table 10). On the other hand PAHs Naphthalene, Acenaphthylene, Acenaphthene, Fluorene, Phenathrene and Fluoranthene in Abu Zenima 2 and Abu Zenima 3 sites were recorded 122.5, 64.22, 73.25, 28.36, 33.59 and 40.22ng/g dw respectively;155.25, 86.49, 112.35, 43.62, 55.93 and 79.24ng/g dw respectively as compared to Maximum allowable Concentrations (Table 10). Table 9 showed also the concentration in the costal sediments for PAHs Naphthalene, Acenaphthylene, Acenaphthene, Fluorene, Phenathrene and Fluoranthene at Abu Rudeis 1 34.5, 14.3, 22.73, 9.44, 9.55 and 13.62ng/gdw respectively. By the way Abu Rudeis 2 and Abu Rudeis 3 sites were recorded 62.34, 33.52, 42.29, 15.79, 15.64 and 26.73ng/gdw respectively; 92.57, 37, 65.43, 22.43, 29.77 and 44.82ng/gdw respectively. These results was found to be in agreement with that observed by Hussain et al. [39], who observed the relatively high concentrations of Naphthalene, Acenaphthylene, Acenaphthene, Fluorene, Phenathrene and Fluoranthene at certain locations Suez Gulf Abu Zenima sites. In the present study PAHs individuals for sediment at Abu Zenima sites Naphthalene, Acenaphthene, Acenaphthyle and Fluoranthene were recorded highly concentration than other PAHs individuals. In this study and when the results compared to the national environmental standard, it was noticed that the low concentration in total and individuals PAHs. This result was found to be in agreement with that observed by El Nemr [40] who recorded that PAHs in the sediments of the Suez Gulf may produce adverse effects on certain organisms living in and coming into contact with the sediments. However, the individual PAH concentrations in this study were lower than the national sediment quality criteria proposed by the USEPA [41] for fluoranthene (3000ngg-1), acenaphthylene (2400ngg-1) and phenanthrene (2400ngg-1).
Table 11: Distribution and concentration levels of PAHs found in marine organisms of different sampling sites in Abu Rudeis coastline.
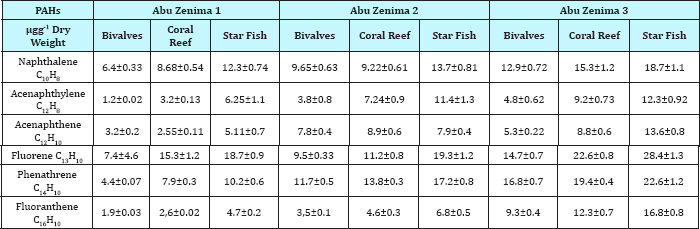
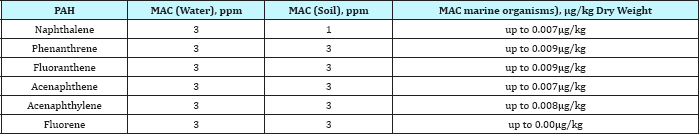
The present work also monitoring the marine organisms (Bivalves, coral reef and starfish) in coastline at Suez Gulf in Abu Zenima and Abu Rudeis sites. Table 11 & 12 showed the distribution and concentration levels of PAHs individuals in Abu Zenima and Abu Rudeis sites. Table 11 showed the increased PAHs individuals, Naphthalene, Acenaphthylene, Acenaphthene, Fluorene, Phenathrene and Fluoranthene in starfish and coral reef than Bivalves. On other hand PAHs individuals recorded highly concentration in Abu Zenima 3 and Abu Zenima 2, than Abu Zenima 1. Where levels was recorded in Abu Zenima1 for concentrations of Naphthalene, Acenaphthylene, Acenaphthene, Fluorene, Phenathrene and Fluoranthene in Bivalves 6.4, 1.2, 3.2, 7.4, 4.4 and 1.9|igg-1 dry weight respectively. On the other hand coral reef and starfish recorded highly concentration than Bivalves, where levels reaching to 8.68, 3.2, 2.55, 15.3, 7.9 and 2, 6|igg-1 dry weight respectively; 12.3, 6.25, 5.11, 18.7, 18.7 and 4.7|igg-1 dry weight respectively. The Bivalves, coral reef and starfish in Abu Zenima 3 and Abu Zenima 2 recorded highly levels in concentrations for Naphthalene, Acenaphthylene, Acenaphthene, Fluorene, Phenathrene and Fluoranthene, where Bivalves recorded in Abu Zenima 2 9.65, 3.8, 7.8, 9.5, 11.7 and 3.5|igg-1 dry weight respectively. Also coral reef and starfish were recorded in Abu Zenima 2 9.22, 7.24, 8.9, 11.2, 13.8 and 4.6|igg- 1 dry weight respectively; 13.7, 11.4, 7.9, 19.3, 17.2 and 6.8|ig g-1 dry weight respectively. On the other hand Bivalves were recorded in Abu Zenima 3 for Naphthalene, Acenaphthylene, Acenaphthene, Fluorene, Phenathrene and Fluoranthene 12.9, 4.8, 5.3, 14.7, 16.8 and 9.3|igg-1 dry weight respectively .The coral reef and starfish also recorded15.3, 9.2, 8.8, 22.6, 19.4 and 12.3|igg-1 dry weight respectively; 18.7, 12.3, 13.6, 28.4, 22.6 and 16.8|igg- 1 dry weight respectively. When compared our results with the data of Maximum allowable Concentrations (MACs) USEPA: 1993 in Table 10, where recorded for Naphthalene, Acenaphthylene, Acenaphthene, Fluorene, Phenathrene and Fluoranthene 0.007, 0.008, 0.007, 0.001, 0.009 and 0.009|ig/kg dry weight respectively. The concentration levels of PAHs individuals in Abu Zenima sites were recorded higher than that in Maximum allowable Concentrations (MACs) USEPA for PAHs in marine organisms [41].
Table 12: Maximum allowable concentrations (MACs) of PAHs in water, soil and marine organisms [41].
These results was found to be in agreement with Al [42], who was observed the accumulation of total PAHs were more pronounced in the tissues of higher lipids contents, which increase in the large and old individuals, and they found that the coral reef and starfish comes second regarding to their PAHs accumulations ratios after the dwelling fish species. Size of the marine organisms may be not only contributed to change the accumulation factors responsible for the quantities of pollutants such as time exposure and lipids contents but also in the factors responsible for the pollutants selectivity. For example, [43] demonstrated that coral reef and starfish of greater size accumulated PAHs of low molecular mass, whereas the smaller mussels had accumulated greater concentrations of high molecular mass PAHs Metabolism may explain this pattern, because it is suspected that PAH of High Molecular Mass (HMW) are more rapidly metabolized than Low Molecular Mass (LMW) due to differences in enzyme affinity [44].
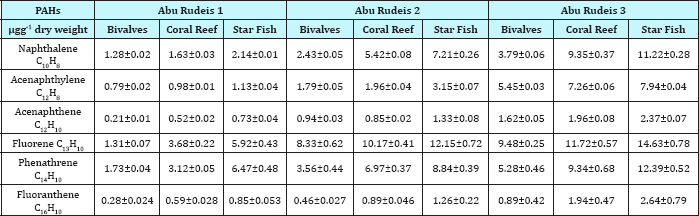
The increased of concentration levels of PAHs individuals in Bivalves, coral reef and starfish at Abu Zenima were be attributed to the presence of many petroleum industrial marine plates in this region at south canal. Also this petroleum plates discharge some oils into the water plus to their incomplete fuel combustion emissions [45]. On the other hand Table 12 showed the low level concentration of PAHs individuals in Abu Rudeis sites as compared to Abu Zenima sites in Table 11 and Maximum allowable Concentrations (MACs) USEPA [41] for PAHs in marine organisms in Table 10. The Naphthalene, Acenaphthylene, Acenaphthene, Fluorene, Phenathrene and Fluoranthene were recorded in Bivalves at Abu Rudeis1 1.28, 0.79, 0.21, 1.31, 1.73 and 0.28|igg-1 dry weight respectively. The impact of PAHs individuals and TE- NORM 238U, 232Th and 40K in marine organisms make dangerous effects on marine ecosystem. These effects were to be attributed for spreading the petroleum marine plates in these sites and areas. On the other hand many of these petroleum industries do not have following environmental regulations and marine protection standards during process for removal petroleum waste. Also during the process of oil transportations and drilling for sludge and oil tanks. The accumulation of double effects of and also many effects of hazardous materials like TE-NORM 238U, 232Th ,40K and PAHs individuals may be devastation the marine ecosystems in red sea coastline and Gulf of Suez.
Conclusion
The concentrations of natural radioactive series nuclides varied widely within the oil fields and from one oil field to another through spectrum of this study area. This study revealed the correlation between the effects of naturally occurring radioactive materials (NORM) 238U, 232Th and 40K on marine ecosystem. In this study, the increased of 238U, 232Th and 40K in sea water and sediments were related to increased in PAHs individuals. The 238U, 232Th and 40K in Abu Zenima 3 and Abu Zenima 2 sites were recorded higher concentration than Abu Zenima 1. Also Abu Rudeis 3 and Abu Rudeis were recorded highly activity than site of Abu Rudeis1. The levels of 238U, 232Th and 40K in sea water and sediments at Abu Zenima and Abu Rudeis were parrled and synchronized with levels of 238u, 232Th and 40K in sea water and sediments at Abu Zenima PAHs individuals in both sites. The study showed the increased level concentrations of 238U, 232Th and 40K and PAHs individuals in sea water and sediments Abu Zenima sites than Abu Rudeis sites as compared to international Maximum allowable concentrations. On the other hand this study revealed the effect of (NORM) 238U, 232Th and 40K and PAHs individuals on marine organisms Bivalves, coral reef and starfish on both sites. The data revealed increased concentration of (NORM) 238U, 232Th and 40K and PAHs individuals for starfish and coral reef than bivalves marine organisms for both Abu Zenima and Abu Rudeis sites respectively as compared to international maximum allowable concentrations. Regarding to these data, all petroleum industries in this sites must be take into consideration the roles and standers' of protection for marine ecosystem. On the other hand, the Egyptian government must be applied the regulation for marine environmental protection lows.
References
- Chowdhury MI, Alam MN, Hazari SKS (1999) Distribution of radionuclides in the river sediments and coastal soils of chittagaong, Bangladesh and evaluation of the radiation hazard. Applied Radiation and Isotopes 51(6): 747-755.
- Fisher NS, Fowler SW, Boisson F, Carroll J, Rissanen K, et al. (1999) Radionuclide bioconcentration factors and sediment partition coefficients in arctic seas subject to contamination from dumped nuclear wastes. Environmental Science and Technology 33(12): 1979-1982.
- Egidi P, Hull C (1999) NORM and TENORM: procedures, users, and proposed regulations. In: Health physics society 32nd midyear topical meeting albuquerque, New Mexico, USA, pp. 25-30.
- El Afifi E M, Awwad NS (2005) Characterization of the te-norm waste associated with oil and natural gas production in Abu Rudeis. J Environ Radioact 82(1): 7-19.
- El Afifi EM, Awwad NS, Hilal MA (2009) Sequential chemical treatment of radium species in TENORM waste sludge produced from oil and natural gas production. Journal of Hazardous Materials 161(2-3): 907-912.
- Peterson HT (1983) Terrestrial and aquatic food chain pathways, radiological assessment. In: Till J, Meyer HR (Eds.), A textbook on environmental dose analysis. NUREG/CR3332, ORNL-5968.
- Hong GH, Baskaran M, Molaroni SM, Lee HM, Burger J (2011) Anthropogenic and natural radionuclides in caribou and muskoxen in the Western Alaskan Arctic and marine fish in the Aleutian Islands in the first half of 2000s. Sci Total Environ 4099(19): 3638-3648.
- Khan MF, Wesley SG (2011) Assessment of health safety from ingestion of natural radionuclides in sea foods from a tropical coast, India. Mar Pollut Bull 62(2): 399-404.
- Ademola JA, Ehiedu SI (2010) Radiological analysis of 40K,226Ra and232Th in fish, crustaceans and sediment samples from fresh and marine water in oil exploration area of Ondo state. Nigeria Afr J Biomed Res 13: 99106.
- Yii MW, Zaharudin A, Abdulm KI (2009) Distribution of naturally occurring radionuclides activity concentration in East Malaysian marine sediment. Appl Rad Isot 67(4): 630-635.
- Amin YM, Nick HW (2004) Radiological monitoring of Malaysian oil and gas industries. J Fiz Malays 25: 47-50.
- Omar M, Ali HM, Abu MP, Kontol KM, Ahmad Z, et al. (2004) Distribution of radium in oil and gas industry wastes in Malaysia. Appl Radiat Isot 60(5): 779-782.
- Hallare AV, Lasafin KJA, Magallanes JR (2011) Shift in phytoplankton community structure in a tropical marine reserve before and after a major oil spill event. Int J Environ Res 5(3): 651-660.
- Huang YJ, Jiang ZB, Zeng JN, Chen QZ, Zhao YQ, et al. (201) The chronic effects of oil pollution on marine phytoplankton in a subtropical bay, China. Environmental Monitoring and Assessment 176(1-4): 517-530.
- El-Sikaily A, Khaled A, Nemr AEl, Said TO, Abd Allah AMA (2003) Polycyclic aromatic hydrocarbons and aliphatic in the coral reef skeleton of the Egyptian red sea coast bull. Environ Contam Toxicol 71(6): 12521259.
- Billiard SM, Timme-Laragy AR, Wassenberg DM, Cockman C, Giulio RT (2006) The role of the aryl hydrocarbon receptor pathway in mediating synergistic developmental toxicity of polycyclic aromatic hydrocarbons to Zebrafish. Toxicology Science 92(2): 526-536.
- Ali NA, Ahmed OE, Doheim MM (2014) Evaluation of poly-aromatic hydrocarbons (PAHs) in the aquatic species of Suez Gulf water along El- Sokhna area to the Suez refineries. Environ Monit Assess 186(2): 1261-1269.
- (1986) Annual book of American Society for Testing and Materials. Soil sampling, 11, p. 1.
- (1995) American Public Health Association standard methods for the examination of water and waste water (19th edn), Washington DC, USA.
- Psichoudaki M, Papaefthymiou H, (2008) Natural radioactivity measurements in the city of ptolemais. Journal of Environmental Radioactivity 99(7): 1011-10117.
- Keyser RM (1995) Characterization and applicability of low- background germanium detectors. EG&G ORTEC, Oak Ridge, TN, USA.
- Kabzinski AKM, Cyran J, Juszczak R (2002) Determination of polycyclic aromatic hydrocarbons in water (including drinking water) of Lodz. Polish Journal of Environmental Studies 11(6): 695-706.
- Kostopoulou M, Mylona A, Nikolaou A, Lofrano G, Meric S, et al. (2007) Determination of polycyclic aromatic hydrocarbons in the harbour sediments of mytilene, Greece, In : Proceedings of the 10th Conference on Environmental Science and Technology, Kos, Greece, pp. 723-726.
- Afifi EM, Khalifa SM, Aly HF (2004) Assessment of the 226Ra content and the 222Rn emanation fraction of TE-NORM wastes at certain sites of petroleum and gas production in Egypt. Journal of Radioanalytical and Nuclear Chemistry 260(1): 221-224.
- El Afifi EM, Awwad NS (2005) Characterization of the TE-NORM waste associated with oil and natural gas production in abu rudeis, Egypt. Journal of Environmental Radioactivity 82(1): 7-19.
- El Afifi EM, Awwad NS, Hilal MA (2009) Sequential chemical treatment of radium species in TENORM waste sludge produced from oil and natural gas production. Journal of Hazardous Materials 161(2-3): 907-912.
- Uosif MAM, El-Taher A, Abbady GE (2008) Radiological significance beach sand used for climate therapy from Safaga, Egypt. Radiation Protection Dosimetry 131: 331-339.
- Harb S (2008) Natural radioactivity and external gamma radiation exposure at the coastal red sea in Egypt. Radiation Protection Dosimetry 130(3): 376-384.
- Gazineu MH, Hazin CA (2008) Radium and potassium-40 in solid wastes from the oil industry. Appl Radiat Null 66(1): 90-94.
- UNSCEAR (2000) Exposure from natural radiation sources, annex-b. Sources and effects of ionizing radiation. United Nations scientific committee on the effects of atomic radiation. New York, USA.
- White GJ (1992) Naturally occurring radioactive materials (NORM) in oil and gas industry equipment and wastes, Washington DC, USA.
- Botezatu E, Grecea C (2004) Radiological impact assessment on behalf of oil/gas industry. The Journal of Preventive Medicine 12 (1-2): 16-21.
- IAEA (1990) The environmental behavior of radium. Vienna Tech, USA, p. 1310.
- (1999) US environmental protection agency technologically enhanced naturally occurring radioactive materials in the southwestern copper belt of arizona. Washington DC, USA.
- Khan MF, Raj YL, Ross EM, Wesley SG (2007) Concentration of natural radionuclides (40K, 228Ra and 221Ra) in sea food and their dose to coastal adult inhabitants around Kudankulam, Gulf of Mannar, South India. Inter J Low Level Radiat 4: 217-231.
- Florou H (1992) Distribution and behaviour of long lived radionuclides in marine ecosystems (Greece) University of Athens, Greece, p. 253.
- Lowman FG, Rice TR (1971) Accumulation and redistribution of radionuclides by marine organisms. Radioactivity in the marine environment. Washington DC, USA, pp. 161-199.
- Omayma EA, Nabila AA, Sawsan AM, Mamdouh MD (2014) Environmental assessment of contamination by petroleum hydrocarbons in the aquatic species of suez gulf, International Journal of Modern Organic Chemistry 3(1): 1-17.
- Hussain M, Rae J, Gilman A, Kauss P (1998) Lifetime health risk assessment from exposure of recreational users to polycyclic aromatic hydrocarbons. Arch Environ Contam Toxicol 35(3): 527-531.
- Nemr El A, Sikaily El A, Khaled A, Said TO, Abd-Allah AMA (2004) Determination of hydrocarbons in mussels from the Egyptian Red Sea coast, Environ Monit Assess 96(1-3): 251-261.
- USEPA (1993) Proposed sediment quality criteria for the protection of benthic organisma, Environmental Protection Agency, Washington DC, USA.
- El Deeb KZ, Said TO, El Naggar MH, Shreadah MA (2007) Distribution and sources of aliphatic and polycyclic aromatic hydrocarbons in surface sediments, fish and bivalves of abu qir bay (Egyptian Mediterranean sea). Bull Environ Contam Toxicol 78: 373-379.
- Maioli OLG, Rodrigues KC, Knoppers BA, Azevedo DA (2010) Polycyclic aromatic and aliphatic hydrocarbons in Mytella charruana, a bivalve mollusk from Mundau Lagoon, Brazil. Microchemical Journal 96(1): 172-179.
- Schnell JV, Gruger EH, Malins DC (1980) Monooxygenase activities of coho salmon (Oncorbynchus kisutch) liver microsomes using three polycyclic aromatic hydrocarbons substrates, Xenobiotica 10(3): 229-234.
- Nassar HF, Tang N, Kameda T, Toriba A, Khoder MI, et al. (2011) Atmospheric concentrations of polycyclic aromatic hydrocarbons and selected nitrated derivatives in greater cairo, Egypt. Atmospheric Environment 45(39): 27352-27359.
© 2018 Kh M Zakaria. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






