- Submissions
Full Text
Environmental Analysis & Ecology Studies
Isotherm Modeling and Thermodynamic Study of the Adsorption of Toxic Metal by the Apricot Stone
Moussa Abbas1*, Tounsia Aksil1 and Mohamed Trari2
1Laboratory of Soft Technologies and Biodiversity, M'Hamed Bouguerra University of Boumerdes, Algeria
2Laboratory of Storage and Valorization of Renewable Energies, University of Science and Technology Houari Boumediene, Algeria
*Corresponding author: Moussa Abbas, Laboratory of Soft Technologies and Biodiversity, Faculty of Science, M'Hamed Bouguerra University of Boumerdes, Algeria
Submission: November 03, 2017; Published: December 14, 2017

ISSN: 2578-0336 Volume1 Issue1
Abstract
The adsorption of Lead onto apricot stone activated carbon (ASAC) in a batch adsorber has been studied. The adsorbent was characterized by FTIR, BET, X-fluorescence, X-ray diffraction and SEM. The effects of contact time, initial pH, agitation speed, adsorbent dosage and initial dye concentration on the Lead adsorption by the ASAC have been studied. Lead removal was seen to increase with increasing contact time until equilibrium and initial dye concentration, and the adsorption capacity of ASAC selected to follow the adsorption process. Batch adsorption experiments were first undertaken to assess the effect of various parameters on the removal efficiency of Lead. It was observed that under optimized conditions up to 166.813mg/g at 25 oC at pH 8 could be removed from solution. Kinetic parameters; rate constants, equilibrium adsorption capacities and correlation coefficients, for each kinetic equation were calculated and discussed. It was shown that the adsorption of Lead onto ASAC could be described by the pseudo second-order equation. The experimental isotherm data were analyzed using the Langmuir, Freundlich and Temkin equations. Adsorption of Lead onto ASAC followed the Freundlich isotherm. The evaluation of thermodynamics parameters indicated respectively the spontaneous and endothermic nature of the reaction and the chimisorption of the sorption process.
Keywords: Apricot stone; Heavy metal; Isotherm; Removal; Thermodynamic
Introduction
The effects of heavy metals like lead, mercury, copper, zinc and cadmium on the human health have been extensively studied and well documented. Excessive ingestion of such metals can cause accumulative poisoning, cancer, nervous system damage. We are interesting by lead which is ubiquitous in the environment and hazardous at high level and its location in the world is reported in. It is a general metabolic poison and enzyme inhibitor and can accumulate in bones, brain, kidney and muscles. Longterm drinking water containing lead causes serious disorders, like anemia, kidney disease and mental retardation. Effluents contaminated by heavy metals are commonly produced from many industrials processes and the residues in contaminated habitats may accumulate in microorganisms, aquatic flora and fauna, which in turn, enter into the human body through the food chain, thus resulting in health problems . So its removal from aqueous medium is necessary because of frequent appearance of this metal in wastewaters from industrial activities. Lead is a common industrial metal that has become widespread in air, water and soil Table 1.
Table 1: Location of lead in the world in 2004.
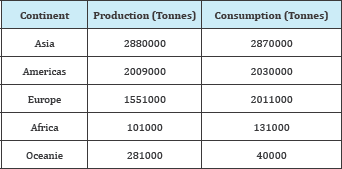
Lead is widely used in the storage batteries, gasoline additives and other chemicals, ammunition (shot and bullets), solder, and other uses and the world production exceeds 3 million tons per year. The presence of heavy metals in the aquatic environment has been of great concern for the scientists and engineers because of their increased discharge, toxic nature, and other adverse effects on receiving waters. In this respect, the excessive utilization of lead has dramatically raised its concentration in blood. In order to insure a better quality of life and protect the environment, removing lead from industrial wastes is of vital importance. Unlike organic compounds, lead is non-biodegradable and, therefore, must be removed from water Agricultural by-products exist in large amounts and about 20.000 tonnes of apricot stones per year are produced in Algeria [1], which represents consequently a solid pollutant to the environment.
Over the past, these by-products were used as fuel in rural areas but now the preparation of activated carbon is considerably encouraged. Apricot stone is a cheap precursor for activated carbon source. Therefore, it is important to evaluate its performance as adsorbent. The advantages of the adsorption reside in the simplicity of the operation, low cost compared to other separation methods and no sludge, the adsorption on activated carbon is an efficient and economic processes. Therefore, this study deals with the adsorption ability of ASAC [2-5] for the removal of lead from synthetic aqueous solutions. The influence of the operating parameters such as initial Pb2+ concentration, pH, temperature, adsorbent dosage, particle size and contact time on ASAC is explored [6].
Experimental
Batch mode adsorption studies
The effects of the experimental parameters such as, the initial Pb2+ concentration (20-100mg/L), pH (2-14), adsorbent dosage (5-45g/L) and temperature (298-323K) on the adsorptive removal of Pb2+ is studied in batch mode for a specific period of contact time (0-90min). The stock solution is prepared by dissolving the accurate amount of PbSO4.7H2O (99%, Merck) in distilled water and other solutions are prepared by dilution. pH is adjusted with HCl (0.1) or NaOH (0.1M). For the kinetic studies, desired quantity of ASAC is contacted with 20mL of Pb2+ solutions in Erlenmeyer flasks. The flasks are then placed on a rotary shaker at 250rpm and the samples are taken at regular time intervals and centrifuged (3000rpm, 10min). The Pb2+ content in the supernatant is determined using Flame Atomic Absorption Spectrometry (FAAS, model Perkin Elmer 2380). The amount of Pb2+ adsorbed on activated carbon qt (mg/g) is calculated by using the following equation (A1):

Where C0 is the initial Pb2+ concentration and Ct the Pb2+ concentrations (mg/L) at any time, V the volume of solution (L) and m the mass of the activated carbon (g). The Pb2+ removal percentage is calculated from the following equation (A2):
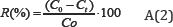
Adsorption kinetics
The kinetic study is important for the adsorption process because it describes the uptake rate of adsorbate, and controls the residual time of the whole process. Two kinetic models namely the, pseudo first order and pseudo second-order are selected in this study for describe the adsorption process. The pseudo first order equation is given by:
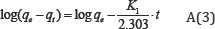
While the pseudo second order model is given by:
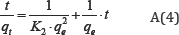
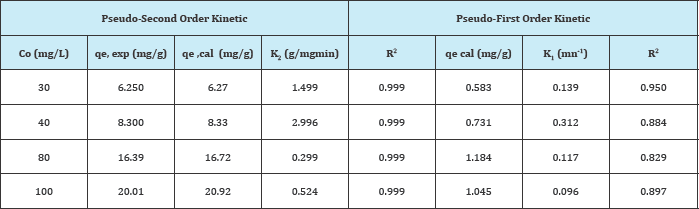
Where qt (mgg-1) is the amount of ions adsorbed on ASAC at the time t (min). K1 (min-1) and K2 (g mg1min-1) are the pseudo-first order and pseudo-second order kinetics constants respectively. For the pseudo-first-order kinetic, the experimental data deviate from linearity, as evidenced from the low values of qe and Co. Therefore, the pseudo-first order model is inapplicable for this system. By contrast, the correlation coefficient and qecal determined from the pseudo-second order kinetic model are in good agreement with the experimental results Table 2.
Table 2: Pseudo-first order and pseudo-second order kinetic model constants.
Adsorption isotherms
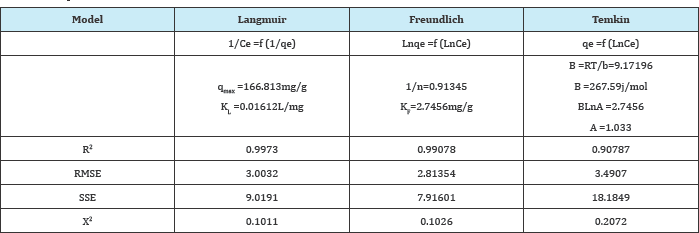
The shape of the isotherms is the first experimental tool to diagnose the nature of a specific adsorption. The isotherms are generally classified in four main groups: L, S, H, and C shapes according to of Giles et al [7]. In our case the isotherm of Pb2+ on ASAC displays an L shape [8]. The initial part ofthe L curve indicates small interaction between Pb2+ and the adsorption sites at low concentrations but when the concentration Co in the liquid phase increases, the adsorption occurs more readily. Such behaviour is due to a synergistic effect with the adsorbed Pb2+, facilitating the adsorbate-adsorbate. The constants parameters are represented adsorption of additional ions as a result of attractive interactions in Table 3.
Table 3: Sorption isotherm coefficients for different models.
Conclusion
The present study has shown that the activated carbon prepared from apricot stone can be employed as effective and low cost adsorbent for the removal of Pb2+ in aqueous solution. Increasing the initial concentration and pH led to an improved adsorption capacity of ASAC. The Freundlich model provides the best fit of the equilibrium adsorption data with a maximum adsorption capacity of 166.813mg/g at pH ~8. The positive thermodynamic parameters indicate that the Pb2+ adsorption onto ASAC is endothermic and not spontaneous. The Pb2+ uptake follows the pseudo-second-order kinetic model, which relies on the assumption that the chemisorption is the rate-limiting step. The enthalpy (Ho, 40kJ/mol) clearly indicates that Pb2+ is strongly attached to the adsorbent surface by forming chemical bond and tends to find sites that maximize their coordination number with the surface.
The results of the present investigation showed that ASAC is a potentially useful adsorbent for the adsorption of metals, an issue of environmental concern and the natural abundance of this food waste can provide an adsorption medium which contributes to the treatment of wastewater. The comparison of the adsorption capacity of the prepared adsorbent with other adsorbents shows its attractive properties from industrial and economic interests. The study in tiny batch gave rise to encouraging results, and we wish to achieve the adsorption tests in column mode under the conditions applicable for the treatment of industrial effluents. This work has been undertaken in the field of the environment to evaluate the application potential of activated carbon prepared from apricot stone as a low-cost adsorbent for the removal of toxic pollutants. Pollutant wastewater has long been a major environmental problem all over the world. The problem is further aggravated by rapid industrialization, population growth and unskilled use of natural water resources.
References
- (2009) FAO Annuaire de la production Ed. Food and Agriculture Organization of the United Nations, Italy.
- Abbas M, Kaddour S, Trari M (2014) Kinetic and equilibrium studies of cobalt adsorption on apricot stone activated carbon. J Ind and Eng Chem 20(3): 745-751.
- Abbas M, Trari M (2015) Process Safety and Environmental Protection 98: 436-446.
- Abbas M, Cherfi A, Aksil T, Trari M (2015) J Envi Analy Toxyco 2: 1-8.
- Abbas M, Cherfi A, Kaddour S, Aksil T (2015) Desalination and water treatment, pp. 1-12.
- Abbas M, Aksil T (2017) Research and Reports in Medecine 1(1): 1-18.
- Giles GH, MacEwan TH, Nakhwa SN, Smith D (1960) J Chem Soc 10: 3973-3993.
- Abbas M, Aksil T (2017) Journal of Materials Processes and Environment 5(1): 1-10.
© 2017 Moussa Abbas, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






