- Submissions

Full Text
Determinations in Nanomedicine & Nanotechnology
Enamine-Based Micellar Asymmetric Organocatalysis in Aqueous Media
András A Gurka*
Department of Organic Chemistry, University of Szeged, Hungary
*Corresponding author:András A. Gurka, Department of Organic Chemistry, University of Szeged, Gyík Str. 20/A, 1/3, 6723, Szeged, Hungary
Submission: March 17, 2023;Published: June 15, 2023

ISSN: 2832-4439 Volume3 Issue2
In light of fundamental challenges of green organocatalysis, possible mechanisms of asymmetric aldol reactions catalyzed by amphipathic proline derivatives in aqueous media are considered to explain the dual stereocontrol stimulated by varying only achiral components. At the suitable reaction conditions in aqueous media (catalyst type and concentration, ionic strength, temperature, etc.) Amphipathic L-hydroxyproline derivative organocatalysts can form micelles, which function as nanoreactors with localization of hydrophobic species in the core, thus allowing the reagents to concentrate at the interface, where the enamine-based asymmetric aldol reaction takes place. The proposed different micellar transition states explain the explored dual stereocontrol stimulated by changing the pH of aqueous solution. In these structures the concerted bond reorganization is mediated with explicit participation of a water molecule, which is the sixth member of the stereoselectivity determining chair-like ring.
Keywords: Aldol reaction; Critical micelle concentration; Transition state; Dual stereocontrol; Nanoreactor; Asymmetric organocatalysis; Enantioselectivity; Zwitterionic amino acid
Introduction
Nowadays, asymmetric organocatalysis has become an alternative and complementary tool in the synthesis of chiral molecules compared to traditional catalysis by enzymes and chiral metallocomplexes. Moreover, organocatalysts are usually cheaper and easier to produce, and have the potential to make synthetic routes greener. For realizing this potential, it is very important to use water as the reaction medium instead of aromatic, polar aprotic and halogenated organic solvents. However, real homogeneous organocatalytic reactions in water are not common. Homogeneous organocatalytic reactions carried out in water-polar organic solvent mixtures are more typical [1-3]. Under true aqueous conditions, when green chemistry has priority, the organocatalytic systems are usually heterogeneous. As regards the role of water in asymmetric organocatalytic reactions, some debates were in the literature about how to consider these reactions when water was used as reaction medium. The terms “in water”, “on water” and “in the presence of water” were discussed [4-9]. C.F.Barbas III and co-workers used amphipathic chiral ammonium salt organocatalyst at large excess of water (brine). The amphipathic catalyst performed very well in the aldol reaction [10] and Michael addition [11]. Authors supposed that reactions proceed efficiently in the concentrated organic phase with excluding water molecules from this phase. Y. Hayashi and co-workers developed an amphipathic silylated L-hydroxyproline, which performed efficiently in the aldol reaction of cyclohexanone with different aldehydes in the presence of water [12,13]. In the presence of large amount of water o/w emulsion was formed and, according to the opinion of the authors, highly hydrophobic catalyst and reagents form an organic phase, in which the enantioselective aldol reaction proceeds. Authors emphasized that the reaction does not proceed at the interface of the aqueous and organic phase, in spite of the formation of an emulsion. Contrary to this, C. Li and co-workers suggested another explanation, studying in the presence of water the aldol reaction of cyclohexanone with aromatic aldehydes catalyzed by amphipathic L-hydroxyprolines. According to their work, w/o and o/w emulsion systems are formed with uniform distribution of amphipathic chiral catalyst molecules in the interface of the emulsion droplets, thus significantly enhancing the reactivity and stereoselectivity of the reaction [14].
Taking into consideration the fundamental challenges in the field of asymmetric organocatalysis, my aims were to realize stereocontrol in the asymmetric aldol reactions catalyzed by L-amino acid derivatives in aqueous media by varying only achiral components, and to clarify the mechanism of the explored inversion of enantioselectivity (dual stereocontrol) in these reactions.
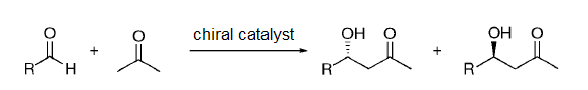
Dual stereocontrol was observed in the amphipathic proline derivatives catalyzed asymmetric aldol reactions between different aldehydes and acetone carried out in aqueous media (Scheme 1) [15,16].
Scheme 1:Asymmetric aldol reaction between different aldehydes and acetone.
Materials and Methods
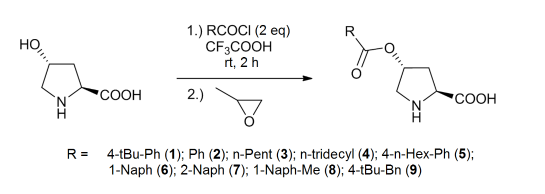
In the reactions commercially available catalysts (L-proline, L-hydroxyproline, O-benzyl-Hyp-HCl) and catalysts newly synthesized by methods known from the literature (O-(4-tertbutylbenzoyl)- Hyp, O-benzoyl-Hyp, O-caproyl-Hyp, O-myristoyl- Hyp, O-(4-hexylbenzoyl)-Hyp, O-1-naphthoyl-Hyp, O-2-naphthoyl- Hyp, O-(1-naphthylacetyl)-Hyp, O-(4-tert-butylphenylacetyl)-Hyp, O-1-naphthoyl-Hyp-methyl-esther, O-benzyl-Hyp) were used. The hydroxyproline derivatives were synthesized by the following method (Scheme 2) [15,16].
Scheme 2:Method of synthesis of L-hydroxyproline derivative catalysts.
Different physico-chemical methods of analysis (1H NMR-,13C NMR-spectroscopy, ESI-MS, melting point measurements) were applied for identification and purity control of the synthesized chiral catalysts. Chiral gas, thin layer and column chromatography, IR-, NMR-spectroscopy and optical rotation measurements were used in the process of analysis of the aldol reaction products.
Brief Summary of Experimental Result and Discussion
In the course of research it was found that water soluble root catalysts (L-proline and L-hydroxyproline) were not active in the reactions, and only the newly synthesized amphipathic derivatives have shown good conversions, selectivities and enantioselectivities [15,16]. These results are explained by phase separation in aqueous media in case of water soluble catalysts, whereas the amphipathic catalysts can provide the conditions when the active center of chiral catalyst and reagents with different water affinity getting close to each other. Because in most experiments the concentration of the used amphipathic L-hydroxyproline derivative catalysts is ~50- 100mM [15,16], which is of the same order of magnitude as Critical Micelle Concentration (CMC) of such tensides, the amphipathic organocatalysts can form micelles. To promote the formation of micelles and accordingly to improve the efficiency of the surface active catalysts, it was important to increase the ionic strength using brine with the required salt concentration.
As a result, it was found that in the solution of an acidic salt (ammonium chloride) in all cases the (R) aldol products were formed in excess, similar to that in organic solvents, with high selectivities and enantioselectivities and moderate conversions. Contrary to this, in the solution of basic salts (alkali metal carboxylates, quaternary ammonium carboxylate) in most of the aldol-reactions the (S) products were formed in excess with excellent conversions and selectivities and with low to moderate enantioselectivities [15,16].
The explored phenomenon is giving the possibility to control
the stereoselectivity of asymmetric aldol reactions catalyzed
by amphipathic L-amino acid derivatives in aqueous media and
according to the recently proposed new universal mechanism [17],
it can be explained with different structures of micellar transition
state, where concerted bond reorganization is mediated with
explicit participation of a water molecule in the stereoselectivity
determining chair-like six membered ring. It is important to note
that only micelle fragments were considered in the mentioned
model. The explanation of dual stereocontrol with two-dimensional
representation of whole micelle structure is as follows:
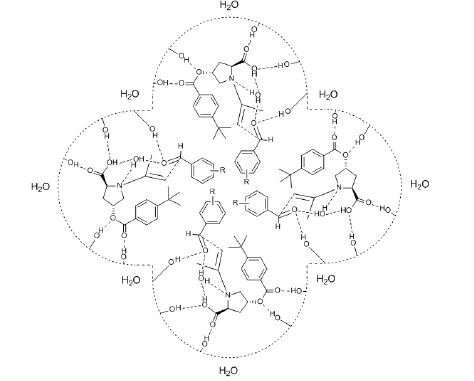
1. Under acidic conditions (concentrated solution of
ammonium chloride) at the interface of the micelle the
carboxylic hydrogen spatially directs the aldehyde through
H-bonding so that the Re-face of the aldehyde is attacked by the
enamine, thus providing the formation of (R) product in excess
(Figure 1).
Figure 1:Micellar transition state of asymmetric aldol reaction between aromatic aldehyde and acetone carried out in the solution of acidic salt.
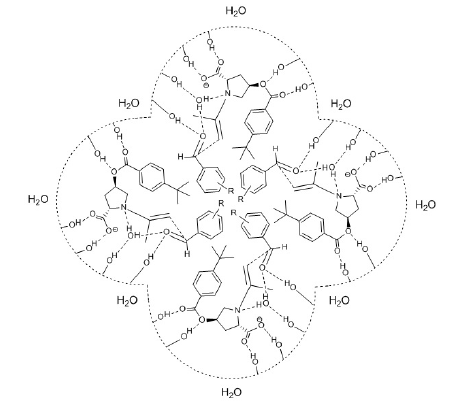
2. In the solution of basic salts (alkali metal carboxylates, quaternary ammonium carboxylate), when the catalyst is lacking the acidic proton and when the carboxylate group of the amino acid acts at the interface of the micelle mainly as a micelle stabilizer, the Si-face of the aldehyde may be more easily attacked by the enamine, resulting in formation of the opposite enantiomer ((S) product is formed in excess) (Figure 2).
Figure 2:Micellar transition state of asymmetric aldol reaction between aromatic aldehyde and acetone carried out in the solution of basic salt.
In the concentrated solution of neutral salt (NaCl), when the amphipathic organocatalyst and the appropriate enamine are predominantly zwitterionic, (S) product is formed in excess. In this case at the interface of the micelle the ammonium proton attaches the lone electron pair of oxygen of the explicit water molecule and, as a result, only single proton of water molecule will be transferred in the transition state (Figure 3).
Figure 3:Micellar transition state of asymmetric aldol reaction between aromatic aldehyde and acetone carried out in the solution of neutral salt.
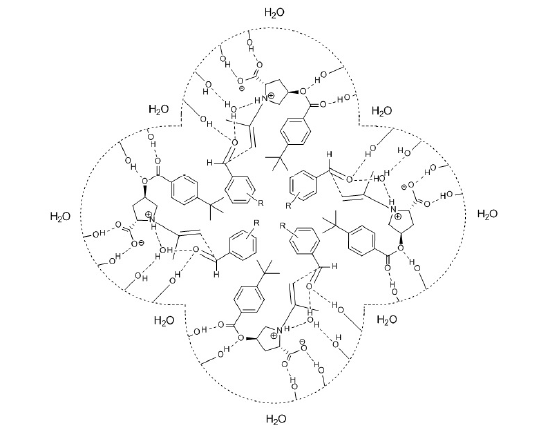
According to proposed mechanism the hydrophobic aldehyde is localized in the core of micelles see (Figure 1-3), thus allowing the reagents to concentrate at the interface near the active center of catalyst. The nanoscale dimensions of micelles (usual size ~5- 100nm) and aqueous character of reaction media make it possible to consider such assemblies as micellar nanoreactors for green asymmetric catalysis.
Conclusion
Micellar model of transition state is proposed for the enaminebased asymmetric aldol reactions catalyzed by amphipathic proline derivatives in aqueous media. In compliance with the offered mechanism, micelles operate as nanoreactors with localization of hydrophobic species in the core of micelles, thus allowing the reagents to concentrate at the interface near the active center of catalyst. The inversion of enantioselectivity stimulated by changing the pH of aqueous solution is explained with different structures of micellar transition states, where concerted bond reorganization is mediated with explicit participation of a water molecule in the stereoselectivity determining chair-like six membered ring.
References
- Dickerson TJ, Lovell T, Meijler MM, Noodleman L, et al. (2004) Nornicotine aqueous aldol reactions: Synthetic and theoretical investigations into the origins of catalysis. The Journal of Organic Chemistry 69(20): 6603-6609.
- Rogers CJ, Dickerson TJ, Janda KD (2006) Kinetic isotope and thermodynamic analysis of the nornicotine-catalyzed aqueous aldol reaction. Tetrahedron 62(2-3): 352-356.
- Ray M, Parashari GS, Singh VK (2009) Highly enantioselective organocatalytic syn- and anti-aldol reactions in aqueous medium. Advanced Synthesis and Catalysis 351(9): 1284-1288.
- Brogan AP, Dickerson TJ, Janda KD (2006) Enamine-based aldol organocatalysis in water: Are they really “All Wet”? Angewandte Chemie International Edition 45(48): 8100-8102.
- Hayashi Y (2006) In water or in the presence of water? Angewandte Chemie International Edition 45(48): 8103-8104.
- Mlynarski J, Paradowska J (2008) Catalytic asymmetric aldol reactions in aqueous media. Chemical Society Reviews 37(8): 1502-1511.
- Pan C, Wang Z (2008) Catalytic asymmetric formation of carbon-carbon bond in the presence of water. Coordination Chemistry Reviews 252(5-7): 736-750.
- Ray M, Singh VK (2009) Organocatalytic reactions in water. Chemical Communications 351(44): 6687-6703.
- Gruttadauria M, Giacalone F, Noto R (2009) Water in stereoselective organocatalytic reactions. Advanced Synthesis and Catalysis 351(1-2): 33-57.
- Mase N, Nakai Y, Ohara N, Yoda H, Takabe K, et al. (2006) Organocatalytic direct asymmetric aldol reactions in water. Journal of the American Chemical Society 128(3): 734-735.
- Mase N, Watanabe K, Yoda H, Takabe K, Tanaka F, et al. (2006) Organocatalytic direct michael reaction of ketones and aldehydes with beta-nitrostyrene in brine. Journal of the American Chemical Society 128(15): 4966-4967.
- Hayashi Y, Sumiya T, Takahashi J, Gotoh H, Urushima T, et al. (2006) Highly diastereo-and enantioselective direct aldol reactions in water. Angewandte Chemie International Edition 45(6): 958-961.
- Aratake S, Itoh T, Okano T, Nagae N, Sumiya T, et al. (2007) Highly diastereo and enantioselective direct aldol reactions of aldehydes and ketones catalyzed by siloxyproline in the presence of water. Chemistry-A European Journal 13(36): 10246-10256.
- Zhong L, Gao Q, Gao J, Xiao J, Li C (2007) Direct catalytic asymmetric aldol reactions on chiral catalysts assembled in the interface of emulsion droplets. Journal of Catalysis 250(2): 360-364.
- Gurka AA, Szőri K, Bartók M, London G (2016) Dual stereocontrol in aldol reactions catalysed by hydroxyproline derivatives in the presence of a large amount of water. Tetrahedron: Asymmetry 27(19): 936-942.
- Gurka AA (2017) Stereocontrol in asymmetric aldol reactions catalyzed by L-amino acid derivatives and immobilized oligopeptides. PhD Thesis-Department of Organic Chemistry, University of Szeged (Szeged), Hungary.
- Gurka AA (2023) New mechanistic approach in the enamine-based asymmetric organocatalysis. Structural Chememistry 34: 83-86.
© 2023 András A Gurka. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






