- Submissions

Full Text
Determinations in Nanomedicine & Nanotechnology
Nano Iron-Loaded Modified Biochar for Removal of Cr(VI) in Aqueous Environment
Xue Wang1, Xiurui Liu1, Yajun Cai1,2, Buyun Wang1,2 and Qi-an Peng1,2*
1College of environmental Engineering, China
2Clean production of textile printing and dyeing engineering research center of the Ministry of Education, China
*Corresponding author:Qi-an Peng, College of environmental Engineering, Clean production of textile printing and dyeing engineering research center of the Ministry of Education, Wuhan Textile University, China
Submission: November 25, 2022;Published: February 03, 2023

ISSN: 2832-4439 Volume3 Issue1
Hexavalent chromium in the water environment is highly toxic and can be enriched through the biological chain, which needs proper management. Bioleaching was used as a biochar modification method to prepare nanoscale iron-modified biochar for the removal of hexavalent chromium from the aqueous environment. The experimental results showed that bioleaching followed by pyrolysis process, the nanoscale iron was successfully loaded onto the biochar and the removal of hexavalent chromium could reach by 93.99% for 100mg/L Cr(VI).
Keywords: Bioleaching; Biochar; Cr(VI); Nano trioxide
Abbreviations:Cr: Chromium; BC: Biochar; ORP: Oxidation Reduction Potential; PMFeBC: Produce Modified Iron-Loaded Biochar; SEM: Scanning Electron Microscopy, XRD: X-ray Diffractometers
Introduction
In recent decades, with the development of industry, anthropogenic activities have released a large amount of Chromium (Cr) into the environment through various production activities, which has caused chromium environmental pollution by seriously exceeding the standard in the environment [1]. Biochar is often used as an inexpensive adsorbent for environmental pollution treatment. For better treatment effect, it is often modified with iron loaded and then used to remove Cr(VI)from water [2]. The common iron modification methods include impregnation [3], ball milling [4], and liquid phase reduction [3], etc. However, these synthesis methods had complicated steps and harsh synthesis conditions. Thiobacillus ferrous oxide, a common strain of microbial metallurgy [5], had efficient iron conversion efficiency and could be tried for iron-carrying modification.
Materials and Method
The original Biochar (BC) was the accessory substance of gas production through a fast pyrolysis of crushed wood in cyclone furnace. Thiobacillus ferrooxidans was domesticated from the sludge by 9K broth. 9K broth composed of 3g/L (NH4)2SO4, 0.1g/L KCl, 0.5g/L K2HPO4, 0.5g/L MgSO4·7H2O, 0.01 g/L Ca(NO3)2, 40.0g/L FeSO4·7H2O. PH was adjusted to 4.00 by H2SO4. All chemicals used in the present study were of analytical grade. The preparation process of modified iron-loaded biochar was conducted in two parts: bioleaching and pyrolysis [6]. The bioleaching process was carried out in 2.5L conical flasks, and the modified precursor (MBC) was obtained by co-cultivating 10g/L Biochar (BC) with Thiobacillus ferrooxidans in 9K medium for 72h. The inoculum of bacteria was 10%, and the incubation temperature was 37 ℃. The changes of ferrous oxidation rate, pH and Oxidation Reduction Potential (ORP) in the medium responded to the growth of Thiobacillus ferrooxidans. After that, the MBC was pyrolyzed in a vacuum tube furnace at the temperature of 1000 °C, the pyrolysis time of 30min, and water vapor flow throughout, with nitrogen as the driving gas to Produce Modified Iron-Loaded Biochar (PMFeBC). Meanwhile, the same conditions of culture and pyrolysis were applied to the control group using 9k medium without the addition of bacteria to produce impregnated iron-loaded modified biochar (FeBC). The materials were digested using digestion solution (HNO3:HClO4=1:1) at high temperature. PMFeBC was characterized by Scanning Electron Microscopy (SEM) and X-ray Diffractometers (XRD) for iron loading on the surface of biochar. Cr(VI) adsorption experiments were performed to compare the modification effect. The experimental conditions were 140rpm, 25 °C, pH=3 and Cr(VI) concentration was 100mg/L
Results and Discussion
Bioleaching was an effective method to modified biochar. As shown in (Figure 1 & 2), Oxidation Rate of Fe2+ and ORP increased over time, and adding biochar didn’t have obvious impact. The change of pH (Figure 3) showed a trend of increasing and then decreasing, which was consistent with the growth trend of ferrous sulfur oxide bacteria. This phenomenon indicated that the oxidation process of Fe2+ to Fe3+ was the process of acid consumption first and then acid production [7]. The addition of biochar had a larger effect on the pH change, probably because of the buffering effect of biochar itself. In (Table 1), it could be seen that the iron content on biochar gradually increased with the growth of Thiobacillus ferrooxidans. According to the XRD characterization results (Figure 4), the loading of iron by the bioleaching process is mainly carried out through the production of the jarosite [8]. After high temperature pyrolysis, jarosite on the surface of biochar was transformed into Fe3O4 through dehydration and desulfurization [9]. SEM (Figure 5) showed that jarosite on the surface of MBC was in a more regular multifaceted prismatic shape with particle size ranging from 1um- 0.3um. From (Figure 6), it could be observed that the biochar structure was fractured after pyrolysis and the Fe3O4 crystal shape was more regular and the particle size could reach below 500nm, and the iron content of the material could reach 294.86mg/g.
Figure 1:Oxidation rate of Fe2+ in 9K medium during bioleaching; filled circle Thiobacillus ferrooxidans, filled square Thiobacillus ferrooxidans and biochar.

Figure 2:Oxidation reduction potential in 9K medium during bioleaching; filled circle Thiobacillus ferrooxidans, filled square Thiobacillus ferrooxidans and biochar.

Figure 3:Change of pH in 9K medium during bioleaching; filled circle Thiobacillus ferrooxidans, filled square Thiobacillus ferrooxidans and biochar.
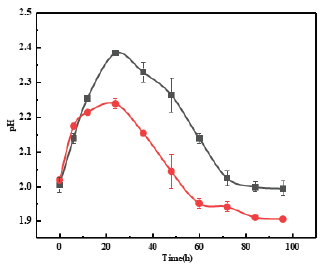
Figure 4:XRD pattern of MBC and PMFeBC.
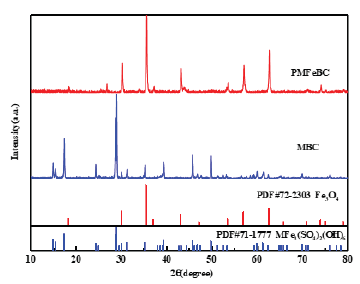
Table 1: Iron content of different materials.

Figure 5:SEM image of MBC.
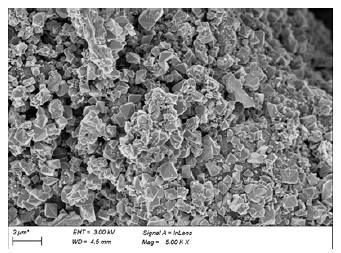
Figure 6:SEM image of PMFeBC.
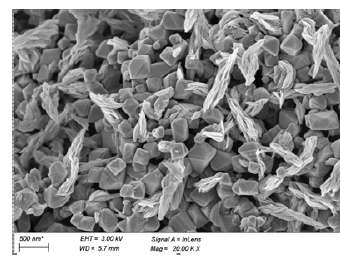
Figure 7:Effect of different materials on removal of Cr(VI); Circle BC; Square MBC; Triangle FeBC; Diamond PMFeBC; Different lowercase letters represented significant differences between treatment groups, p<0.05.
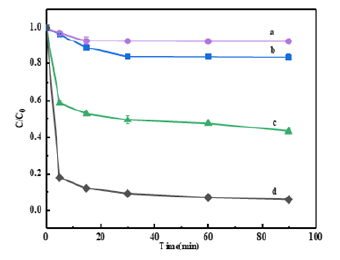
In the Cr(VI) adsorption experiments, the modified material PMFeBC showed a significant removal effect compared with the iron-bearing biochar made by impregnation. It could be seen from (Figure 7) that up to 93.99% removal was achieved at 90min. The reason may be that the surface-loaded ferric tetroxide on biochar reacts with dichromate under acidic conditions to form trivalent chromium.
Conclusion
The experiments used the biological method to modify the biochar with iron-carrying, and produced the Fe-loaded modified biochar with efficient adsorption performance, which had the advantages of green, environmental protection and sustainability.
References
- Sharma N, Sodhi KK, Kumar M, Singh DK (2021) Heavy metal pollution: Insights into chromium eco-toxicity and recent advancement in its remediation. Environmental Nanotechnology Monitoring & Management 15: 100388.
- El-Naggar AL, Mosa A, Ahmed N, Niazi NK, Yousaf B, et al. (2022) Modified and pristine biochars for remediation of chromium contamination in soil and aquatic systems. Chemosphere 303(1): 134942.
- Shaheen SM, Mosa A, Abdelrahman H, Niazi NK, Antoniadis V, et al. (2022) Removal of toxic elements from aqueous environments using nano zero-valent iron- and iron oxide-modified biochar: A review. Biochar 4(1): 1-21.
- Wang K, Sun YB, Tang JC, He J, Sun HW (2020) Aqueous Cr(VI) removal by a novel ball milled Fe0-biochar composite: Role of biochar electron transfer capacity under high pyrolysis temperature. Chemosphere 241.
- Deshpande AS, Kumari R, Rajan AP (2018) A delve into the exploration of potential bacterial extremophiles used for metal recovery. Glob J Environ Sci Manag 4(3): 373-386.
- Liu X, Wang X, Yang W, Yuan F, Wang B, et al. (2022) Impregnating biochar with Fe and Cu by bioleaching for fabricating catalyst to activate H2O2. Applied microbiology and biotechnology 106(6): 2249-2262.
- Feng SS, Yang HL, Wang W (2015) Insights into the enhancement mechanism coupled with adapted adsorption behavior from mineralogical aspects in bioleaching of copper-bearing sulfide ore by Acidithiobacillus sp. RSC Adv 5(119): 98057-98066.
- Zhao KL, Gu GH, Wang XH, Yan W, Qiu GZ (2017) Study on the jarosite mediated by bioleaching of pyrrhotite using Acidthiobacillus Ferrooxidans. Biosci J 33(3): 721-729.
- Zhang B, Zhu L, Liu W, Han J, Jiao F, et al. (2019) Sulfidation and sulfur fixation of jarosite residues during reduction roasting. Metallurgical and Materials Transactions B 50(2): 761-771.
© 2023 Qi-an Peng. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






