- Submissions

Full Text
Determinations in Nanomedicine & Nanotechnology
Plant-Mediated Synthesis of Silver Nanoparticles and Antibacterial Activity on Implicated Biomolecules of Green Spinicia Oleracea Leaf Extract
Mohammed RA1*, Saleh GM2 and Mutlak FA1
1College of Science Department of Physics, Iraq
1College of Science Department of Biology, Iraq
*Corresponding author: Ruaa A Mohammed, College of Science Department of Physics, Baghdad, Iraq
Submission: February 2, 2022;Published: April 20, 2022

ISSN: 2832-4439 Volume2 Issue4
Abstract
This study used green spinach leaf extract for the green synthesis (monocolors) of silver nanoparticles (AgNPs). This search focuses on the optimization of synthesis by evaluating the impact on the each of the size distribution and activity of antibacterial against bacteria (Escherichia coli) of the green spinach leaf extract volume percent. The characterization of AgNP’s was carried out utilizing UVVisible spectrophotometers, size examination of particles, transmission electron microscopes, energy spectrometry with dispersion X rays, Fourier infrared spectrometer transform and x-ray scanning methods. The experimental data show that AgNPs have been produced effectively and size of particle is controlled by the amount of green spinach leaf extract. With the size reaching (5nm, polydispersity indices=0.063nm), the smaller volume percentage creates AgNPs with a spherically formed reach of 20%. The synthesized AgNPs by the spinach leaf extract had a good antibacterial activity especially at the concentration of (500μg/ml) in which the inhibition zone reached 35mm against gram negative bacteria Escherichia coli.
Keywords:Spinicia oleracea extract; AgNP’s synthesis; Antibacterial activity
Abbreviations:ZnONP: Zinc Oxide Nanoparticles; PtNPs: Platinum Nanoparticles; TEM: Transmission- Electron Microscope; XRD: X-Ray Diffractors; SPR: Surface Plasm Resonance; DNA: Deoxyribonucleic Acid; NPs: Nanoparticles
Introduction
The use of plants’ extract for the production of metal nanoparticles is an emerging technology that is extensively explored in the past several years with a view to replacing dangerous and non-renewable chemicals. Green production of nanoparticles in recent years has been an intriguing subject [1]. The fundamental concept of synthesis is the capacity to reducing metal ion precursors by flavonoids and alkaloids. Certain metal and metal oxide nanoparticles, for example silver nanoparticles [2], gold nanoparticles [3,4], Zinc Oxide Nanoparticles (ZnONP), [5] and Platinum Nanoparticles (PtNPs), are created via the redactor of plant extract [6]. AgNP green synthesis production is intriguing since several research have shown synthesized nanoparticles’ features as a function of the plant extract type, content, and synthesis methods. The antibacterial, anti-fungal, anticancer and antioxidant effects of green synthesized AgNPs are claimed to have occurred. AgNPs’ activity, physical, and chemical characteristics are influenced by their shape and form, which are influenced by the plant extract employed, composition, and synthesis technique [7]. Different investigations have been reported over the years on AgNP production utilizing plant extracts. Some of the earlier investigations revealed a green synthesis of AgNPs with the aid of Lantana camarader [8], Parkia speciossa hask pod [9], apple extract [10], Buddleja globosa hope [11] and Azadirachta indica [12]. Some research has shown that the biological/chemical activities of AgNPs are closely linked to the physicochemical properties of AgNPs. Several researches stated in (Table 1) show that the molar ratio is a key parameter for forming the particle size when employing plant extract for AgNO3 synthesis, rather than for the technique used for the manufacture of nanoparticles. An interesting subject is the exploration of various plant extracts for the AgNPs production [13-15].
By reviewing prior research that revealed the usage of red type spinach (Amaranthus Tricolor L.) to provide a secondary metabolite of the chemicals. present research looks at using Green Spinicia oleracea leaf extract as a reducing agent in the manufacture of Ag- NPs. This research focuses on the extraction, optimization, synthesis, and assessment of antibacterial properties of produced AgNPs [16-18].
Materials and Methods
Materials
The analytical grade of all reactants in this investigation is utilized without any additional purification. Silver nitrate (AgNO3) was purchased from (Avonchem limited UK) and Merck-Millipore supplied the distilled water (Germany). Spinicia oleracea green leaf extract was collected in Baghdad, Iraq, from a local market. The soaking process was used to create Spinicia oleracea leaf extract (GSE). Overnight, 50g dry green spinach leaves were soaked in roughly of water (100ml) solvent. GSE was extracted from the combination by filtering the solution. in an oven the soaking extract was then dried at 50 °C and 1 gram of the powder produced after drying was weighed and analyzed in 50ml of distilled water to dilute the extract with diluted silver nitrate in a molar ratio specified for the purpose of research.
Synthesis of AgNPs
Silver nanoparticles have been synthetized by mixing AgNO3- 10-2M with GSE at a constant volume ratio of 20%. The blend was handled for 5 hours at room temperature to guarantee a reduction reaction between Ag+ and Ag0 [19]. The decline study was confirmed by UV-Visible spectroscopy. Analyzer of particle size, infrared microscope and Transmission-Electron Microscope (TEM) Fourier transform was used to further investigate the AgNPs. For these tests, the Particle Size Analyzer HORIBA and the JEOL TEM apparatus were used with a dynamic light dispersion system operating at 120kV. In order to ensure that the single phase of Ag is acquired from synthesis, the Perkin–Elmer spectrometer equipment was used and X-Ray Diffractors (XRD) analysis was carried out using the Shimadzu X6000 equipment, which operated as a radiation source with the Ni filtered CuKα.
Antibacterial activity test of AgNPs
Using the well diffusion experiment, the antibacterial activity of produced AgNPs was determined. Synthesized AgNPs with stock concentration (500μg/ml) and dilutions (250, 125μg/ml) was used to detect the antibacterial activity. The bacterial culture in the Mc- Farland turbidity tube was activated for 18 hours in nutritional broth at 37 pounds in a McFarland turbidity tube with a concentration of 1.5*108 cells/ml. At 18hr, pathogenic bacteria (Escherichia coli) were active at 37LC. Pathogenic bacteria utilizing cotton swab have been injected on sterilized nutrient agar plates. The wells were cut off by using a sterile pipette of a pasture after 5-10 minutes. At each concentration, agNPs solution (100μL) has been applied to the well and incubated for 24 hours at a temperature of 37 °C. Inhibition areas were measured in mm after the incubation period [20].
Results and Discussion
Figure 1:UV-visible AgNP spectrum synthesized.

Figure 2: Particle size distribution of AgNPs.
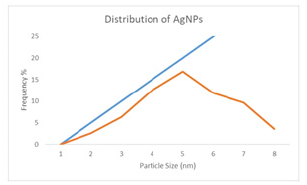
Depicts the UV-visible spectra of produced AgNP at various GSE volumes, (Figure 1). GSE shows specific wavelengths in the range 300-350nm and the strong spectrum 406nm. These spectrums are connected to the presence of RSE anthocyanine and phenolic compounds, which is consistent with previous research [21]. All AgNPs have a maximum wavelength of between 390 and 430nm, which indicates Surface Resonance (SPR). In order to indicate the Surface Plasm Resonance (SPR) absorption range, AgNPs shows a maximum wavelength in the 390-430nm region. The electronic UV-visible light wavelength of the nanosized Ag is related to these SPR absorption bands. Additionally, all samples of AgNPs show the presence of polyphenols or aromatic molecular structures of GSE at peaks at approximately 300-340nm. Given that each AgNP sample demonstrates a single SPR band, the nanoparticles are projected to be spherical, whereas the nanoparticles correspond to the anisotropic molecules, as there are two and more SPR bands [22]. It was proven that the greater GSE size percentage delivers the larger maximum wavelength with the broader area after executing multiple trials to acquire the optimal GSE size % and via the researchers’ earlier investigations. AgNPs-2 produced the most intense spectrum associated with SPR production. The examination of particle size ensures the average distribution of particle size and particle sizes. (Figure 2) displays distribution curves and (Table 2) tabulates the average particle size figures. The GSE volume percent (16.45nm) with lesser polydispersion (PI) value (PI=0.063) was used to achieve the uniform size of particulate matter. The larger the GSE volume, the larger the average particle size and the PI show the widespread size. This behavior is in accordance with the UV spectrum and can lead to greater particle sizes at higher GSE levels [11,23].
Table 1: Some studies on silver nanoparticles biosynthesis.

Table 2: Synthesized AgNP particle size data.

Figure 3: The FTIR spectra of produced AgNPs were compared to those of GSE.
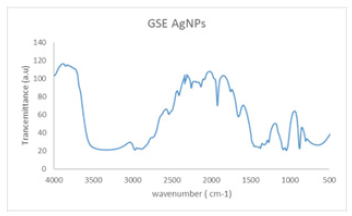
(Figure 3) shows the AgNPs-20% and GSE FTIR spectrums. The presence of (O–H) functional group in GSE samples shows prominent absorbance bands detected at around 3100–3900cm‐1. Other high points at 1661 and 1926, 2538 and 2972cm–1 was detected, respectively, indicating –C=O, -O–-C, and alkane (C–H). C–C and C–N vibrations of the tetrapyrrole ring of chlorophyll, which are connected with a UV–visible vibration, are principally related to the peaks of ν∼1049cm−1 and ν∼1381cm−1. The absorption spectrum found is comparable to the FTIR spinach extract analysis [24]. These findings are comparable to earlier AgNP biosynthetic extract research [8]. (Figure 4) shows the spherical form of nanoparticles between 2-41nm in the image of the spinach extract with AgNP. The findings fit into the distribution of particulate size and UV-visional AgNP spectrum that indicates the nanoparticles’ size range. The particles are also evident when the organic material from the plant extract is covered by a thin coating. The organic cap material aids in the stabilization of nanoparticles. The existence of organic material has been also shown by the X-ray spectrum energy dispersion of AgNPs, which displays Ag, C and O existence. As capping AgNPs, the C and O signals derive from organic GSE molecules. There is no N signal that indicates the lack of AgNO3 since the Ag+ production is completely reduced. The measurement of XRD was conducted in order to ensure the synthesis of the single AgNP material produced. The reflex spectrum of filtered AgNPs is shown in (Figure 5). Four strong peaks are shown in the XRD pattern with 2θ values between 30 and 70. Intense peaks at 2θ point values are in the range of 31.9,43.31, 60.4 and 63.4 (111), (200), (220) and (311) according to JCPDS, silver file 04-078 [25].
Figure 4: TEM image of AgNPs synthesized.
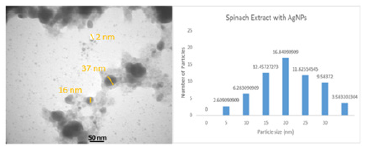
Figure 5: Image of XRD pattern of AgNPs synthesized.
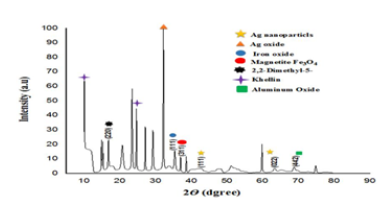
Table 3:Inhibition zone of antibacterial activity test of synthesized GRE AgNPs.

GRE AgNPs have been studied for their antibacterial effects, and the results were presented in (Table 3), which included a comparison of the inhibition areas in the samples for GRE, as well as with amoxicillin as a positive control and with water: ethanol (1:1) as a solvent GRE, where the obtained area indicates that the maximum Antibacterial activity is what was extracted and prepared from a sample of GRE AgNPs. The antibacterial activity increases with the proportion of GRE, which is likewise consistent with the rising particle size average. The volume ratio of silver nanoparticles concentration has no effect on the inhibition zones because it works in principle on the size of the obtained nanoparticles with the effect of the extract on the sizes of these particles, which means that the nanoparticles have no effect on the antibacterial activity at different GRE percentages. These results show that particle size has an influence on antibacterial activity, with smaller particles being more effective [26]. The smaller size aids in more efficient penetration of the bacterium cell membrane for subsequent degradation of sulfur- and phosphorus-containing complexes such as DNA and causing cell death [22].
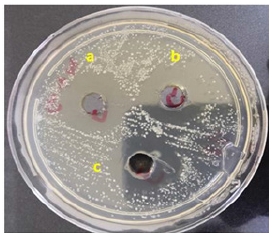
For synthesizing nanoparticles many plant components or complete plants are utilized [27]. Differences in the size or form of the produced nanoparticles in inhibition diameters might be attributed to differing effects on bacterial growth and causing inhibition [25]. Such formulations can be utilized for numerous biotechnological applications and medicinal purposes to destroy harmful microorganisms. The small size and high surface to volume ratio was related to their bactericidal effect of metal nanoparticles, which let them interact with microbial membranes and other contents of the bacterial cell [28]. Antimicrobial feature of the AgNPs are the positive charges and the ionized silver form. These ions develop complications with DNA, notably nucleosides, and other bacterial cell components [29]. Studies have demonstrated that electrostatic interaction exists between positively charged Nanoparticles (NPs) and negatively charged bacterium cells as a bactericidal agent. These NPs build up within bacterial membranes, infiltrate bacterial cells, cause harm, and eventually kill them [30]. Some studies have demonstrated that the silver atoms connect to (-SH) the group of bacterial enzymes, causing stable S-Ag bonds that result in enzyme deactivation, while others have suggested that silver ions entering cells interrupt the base pairs of pyrimidines and purines, causing hydrogen bond interruption between the parallel DNA strands and eventually causing denaturization. The interaction with bacterial cell macromolecules includes the process of electron release and free radical production [31]. Inhibit protein and cell wall production inducing NPs is caused by buildup of precursor envelope proteins, external membrane disruption, and finishes by energy leakage [32]. As shown in (Figure 6) depicts the efficacy of the GRE plant extract with AgNPs on the bacteria used, as well as the antibacterial activity of the GRE AgNPs depending on the size of the silver nanoparticles, which were finally formed with the extract to give an efficacy of bacterial inhibition due to the small size of these nanoparticles and their penetration into the innovative cell membrane, with an effective diameter of up to 32mm.
Figure 6:The antibacterial activity of synthesized AgNPs by GRE plant extract against E. coli. Inhibition zones according to concentration of synthesized AgNPs; a: 35mm at 500μg/ml, b: 32mm at 250μg/ml, c: 30mm at 125μg/ml.
Conclusion
The use of Green Spinach leaf extract as a bio reduction was effectively produced in silver nanoparticles (AgNPs). The analysis of UV-visible Spectrophotometry, FTIR and XRD indicate that Ag- NPs are produced from the full Ag+ reduction of AgNO3 nano-size precursor. The volume percentage of GSE is shown to impact the average and distribution of different particle sizes. The effective capping and stabilizing characteristics of the AgNPs were shown in the FTIR, EDX and SEM analyzes. In addition, the synthesized AgNPs showed antibacterial activity against E. coli, with the tendency to enhance antibacterial activity as a consequence of smaller particle size.
Funding
This work was supported by Science of Physics Dept. & Science of biology Dept. College of Science, University of Baghdad and Ministry of Higher Education and Scientific Research (IQ).
References
- Sharma D, Kanchi S, Bisetty K (2019) Biogenic synthesis of nanoparticles: A review. Arabian J Chem 12(8): 3576-3600.
- Reddy G, Thakur A (2017) Biogenic synthesis of silver nanoparticles using plant waste material. Rasayan J Chem 10(3): 695-699.
- Zeiri Y, Elia P, Zach R, Hazan S, Kolusheva S, et al. (2014) Green synthesis of gold nanoparticles using plant extracts as reducing agents. Int J Nanomedicine 9: 4007-4021.
- Das RK, Borthakur BB, Bora U (2010) Green synthesis of gold nanoparticles using ethanolic leaf extract of Centella asiatica. Mater Lett 64(13): 1445-1447.
- Bala N, Saha S, Chakraborty M, Maiti M, Das S, et al. (2014) Green synthesis of zinc oxide nanoparticles using Hibiscus subdariffa leaf extract: Effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv 5: 4993-5003.
- Siddiqi KS, Husen A, Rao RAK (2018) A review on biosynthesis of silver nanoparticles and their biocidal properties. Nanoscale Res Lett 16: 1-28.
- Sirelkhatim A, Mahmud S, Seeni A, Kaus NHM, Ann LC, et al. (2015) Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro Lett 7(3): 219-242.
- Fatimah I, Indriani N (2018) Silver nanoparticles synthesized using Lantana camara flower extract by reflux, microwave and ultrasound methods. Chem J Moldova 13(1): 95-102.
- Fatimah I (2016) Green synthesis of silver nanoparticles using extract of Parkia speciosa Hassk pods assisted by microwave irradiation. J Adv Res 7(6): 961-969.
- Ali ZA, Yahya R, Sekaran SD, Puteh R (2016) Green synthesis of silver nanoparticles using apple extract and its antibacterial properties. Adv Mater Sci Eng, p. 1-6.
- Carmona ER, Benito N, Plaza T, Recio-Sánchez G (2017) Green synthesis of silver nanoparticles by using leaf extracts from the endemic Buddleja globosa Green Chem Let Rev 10(4): 250-256.
- Ahmed S, Ahmad SM, Swami BL, Ikram S (2015) Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J Radiation Res Appl Sci 9(1): 1-7.
- Shaik MR, Khan M, Kuniyil M, Al-Warthan A, Alkhathlan HZ, et al. (2017) Plant-extract-assisted green synthesis of silver nanoparticles using Origanum vulgare l. extract and their microbicidal activities. Sustainability 10(4): 913-920.
- Rao B, Tang RC (2017) Green synthesis of silver nanoparticles with antibacterial activities using aqueous Eriobotrya japonica leaf extract. Adv Nat Sci: Nanosci Nanotechnol 8(1): 1-9.
- Bagherzade G, Tavakoli MM, Namaei MH (2017) Green synthesis of silver nanoparticles using aqueous extract of saffron (Crocus sativus L.) wastages and its antibacterial activity against six bacteria. Asian Pacific J Tropical Biomed 7(3): 227-233.
- He Y, Wei F, Ma Z, Zhang H, Yang Q, et al. Green synthesis of silver nanoparticles using seed extract of Alpinia katsumadai, and their antioxidant, cytotoxicity, and antibacterial activities. RSC Adv 7(63): 39842-39851.
- Rasheed T, Bilal M, Li C, Nabeel F, Khalid M, et al. (2018) Catalytic potential of bio-synthesized silver nanoparticles using Convolvulus arvensis extract for the degradation of environmental pollutants. J Photochem Photobiol B 181: 44-52.
- Rasheed T, Bilal M, Iqbal HMN, Li C (2017) Green biosynthesis of silver nanoparticles using leaves extract of Artemisia vulgaris and their potential biomedical applications. Colloids Surf B: Biointerfaces 158: 408-415.
- Vasireddy R, Paul R, Mitra AK (2012) Green synthesis of silver nanoparticles and the study of optical properties. Nanomaterials and Nanotechnology 2: 1-8.
- Ghada MS, Shaymaa SN, Abass M (2019) Golden apple snail eggs extract: Biosynthesis of nanoparticles and its antibacterial effect. Research J Pharm and Tech 12(7): 3444-3450.
- Ahliha AH, Nurosyid F, Supriyanto A, Kusumaningsih T (2017) The chemical bonds effect of anthocyanin and chlorophyll dyes on TiO2 for Dye-Sensitized Solar Cell (DSSC). J Phys: Conf Series 909: 1-7.
- Bhakya S, Muthukrishnan S, Sukumaran M, Muthukumar M (2016) Biogenic synthesis of silver nanoparticles and their antioxidant and antibacterial activity. Appl Nanosci 6: 755-766.
- Banerjee P, Satapathy M, Mukhopahayay A, Das P (2014) Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: synthesis, characterization, antimicrobial property and toxicity analysis. Biores Bioprocessing 1: 1-10.
- Sengupta D, Mondal B, Mukherjee K (2015) Visible light absorption and photo-sensitizing properties of spinach leaves and beetroot extracted natural dyes. Spectrochimica Acta-Part A: Mol Biomol Spectr 148: 85-92.
- Salam HA, Rajiv P, Kamaraj M, Jagadeeswaran P, Gunalan S, et al. (2012) Plants: Green route for nanoparticle synthesis. I Res J Biological Sci 1(5): 85-90.
- Bilal M, Rasheed T, Iqbal HMN, Hu H, Zhang X (2017) Silver nanoparticles: Biosynthesis and antimicrobial potentialities. Int J Pharmacol 13(7): 832-845.
- Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, et al. (2007) Antimicrobial effects of silver nanoparticles. Nanotechnology, Biology and Medicine 3(1): 95-101.
- Morones JR, Elechiguerra JL, Camacho A, Holt K, Ramirez JT, et al. (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16(10): 2346-2353.
- Klueh U, Wagner V, Kelly S, Johnson A, Bryers JD (2000) Efficacy of silver-coated fabric to prevent bacterial colonization and subsequent device-based biofilm formation. J Biomed Mater Res 53(6): 621-631.
- Matthew ED, Schaeublin NM, Farrington KE, Hussain SM, Johnson GR (2009) Lysozyme catalyzes the formation of antimicrobial silver nanoparticles. ACS Nano 3(4): 984-994.
- Cao Y, Jin R, Mirkin CA (2001) DNA-modified core–shell Ag/Au nanoparticles. J Am Chem Soc 123(32): 7961-7962.
- Park J, Lim DH, Lim HJ, Kwon T, Choi JS, et al. (2011) Size dependent macrophage responses and toxicological effects of Ag nanoparticles. Chem Commun 47(15): 4382-4384.
© 2022 Mohammed RA. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






