- Submissions

Full Text
Determinations in Nanomedicine & Nanotechnology
Insights of Transdermal Drug Delivery: Prerequisites and Current Strategies
Niharika Lal*
Department of Pharmacy, Metro Group of Institutions, India
*Corresponding author: Niharika Lal, Department of Pharmacy, Metro Group of Institutions, Greater Noida, Uttar Pradesh, India
Submission: November 16, 2021;Published: December 10, 2021

ISSN: 2832-4439 Volume2 Issue3
Abstract
Transdermal Drug Delivery System (TDDS) is an approachable substitute that could minimize as well as avoid limitations correlated with parental and oral drug delivery. The TDDS drug delivery system provides an acceptable and prolonged drug delivery in steady state and minimizes the possibility of peak associated side effects. For controlled drug delivery, utilization of transdermal patches is immensely userfriendly, comfortable and could be easily terminated if any kind of system toxicity arises. The transdermal route provides permeation of drug across the skin and into systemic circulation, avoiding the first pass hepatic metabolism of drugs which in turn increases bioavailability. However, many factors governs the drug delivery from transdermal patch. This reviews give insights for factor governing drug permeation and excipients needed in casting a transdermal patch.
Keywords: Transdermal; Patch; Bioavailability; Drug permeation
Abbreviations: TDDS: Transdermal Drug Delivery System; DMSO: Dimethyl Sulfoxide; PSA: Pressure Sensitive Adhesives; OTC: Over The Counter; LPP: Lipid-Protein Partitioning
Introduction
The penetration of drug across skin through percutaneous delivery is limited by the
highly well-organized structure of stratum corneum which acts as a barrier function for
permeation of drugs. The outermost layer stratum corneum is immensely compelled and
useful for many dynamic drugs [1]. The daily dose of particular drugs that have reached
the market is consistently typically in terms of few milligrams. Moreover, the relationship
between molecular properties and skin permeability, have developed Lipinski rules of 5, that
a drug required potent pharmacological activity, to be an attainable candidate for TDD:
i. The molecular weight of drug should lie in between 400 to 500 daltons.
ii. Balanced lipophilicity (log P, between 2 to 4) is desired.
iii. A consistent solubility both in the non aqueous and aqueous medium is required as the
drug needs to breach the stratum corneum and its absorption is required in the systemic
circulation 4. The number of hydrogen bond donor should be less than 10.
iv. The number of hydrogen bond acceptor should be less than 5 [2].
Despite mentioned drug properties, the penetration of drug across stratum corneum
is the major limiting factor. For an adult person, the skin covers around 15% of total body
weight with a surface area of about 2m2. It is a multilayered organ which is composed of
various histological layers mainly described as epidermis and dermis [3]. The Epidermis is
the outer layer of skin which consists of 95% of keratinocytes cell that has no blood supply
but is nourished by blood vessels in the dermis. The thickness of epidermis is usually 0.5mm-
1mm but depends upon the site for example, it is thick on the palms and soles to provide flexibility and to resist any mechanical injury. The epidermis is
thin on the eyelids to allow maximum movement. The keratinocyte
(major component) along with melanocytes, langerhans cells
and merkel cells (minor components) forms a ‘brick and mortar’
structure. The lower portion of the epidermis is immature and
they rapidly proliferate themselves to form daughter cells to
mortal differentiation that resulted in the development of stratum
corneum. The terminally differentiated keratinocytes called as
corneocytes are responsible for the formation of tight junctions and
named as ‘bricks’, with the nerve of skin. The space between these
bricks is fulfilled by ‘mortar’ that consists of various lipid bilayers of
fatty acids, ceramides, cholesterol esters, and cholesterol. Beneath
the epidermal layer, lies dermis which is 25 to 30 times thicker than
the epidermis.
It composed of a dense network containing specialized proteins
components called collagen and elastic fibers. In comparing to the
epidermis fewer cells with much more fibers are found in the dermis
[4]. The transdermal patch is used for controlled dose delivery
of a drug through the skin over a period of time. The elements of
transdermal patches are backing membrane, drug reservoirs, drug
liners, release membrane, adherents etc. that play a crucial role
in the release of drug through the skin. It is considered that welldesigned
TDDS could supply drug at a rate to sustain the required
therapeutic plasma concentration without much fluctuation or
therapeutic inefficacy. Due to the slow transport of drugs across the
skin, the lag time required to reach steady state fluxes is in hours.
The attainment of an effective level of drug is therefore difficult
without any enhancement of skin permeation [4]. Thorough
strategies were studied to enhance permeabilities of the drug of
stratum corneum in controlled manner. A number of techniques
have been amended to promote transport of a range of drugs across
the skin. These methods involve physical and chemical methods
in which the former provides driving force which act on a drug to
promote its permeation and while later increases skin permeability
by altering or disrupting nature of skin.
There have been many approaches that have been used to
improve but are difficult to predict exact degree of enhancement
of drugs. These approaches range from use of chemical enhancers
to electroporation, iontophoresis and generated ultrasound
waves or synergism of both mechanisms. The limitation to these
enhancement technologies is relevant with skin tolerability. The
skin sensitization differs from one individual to another. It could
be due to use of potent drug or chemical enhancer or polymer that
will be in direct contact with skin. Skin irritation could be detected
from reddening of application site to merely occlusion of skin.
Therefore an early testing for skin irritation studies is necessary
for development and safe marketing of transdermal products [5,6].
Factors Affecting Skin Penetration
There are various factors that affect penetration of drug through skin. This could be due to species variations, ageing of skin, site of application, condition of skin (normal, hydrated or dehydrated), area of application, contact time, physical properties of the penetrant which could depend on lipophillicity of drug or penetration enhancers. However, the molecules could permeate across the SC by three pathways: intercellular, intracellular and by follicular. There should be a deep understanding to study various factors that affect permeability of drug via healthy skin. The local effect could be achieved by dissolving the drug in suitable vehicle that could be applied topically. Most of semisolid formulations were used to achieve local cutaneous effects [7]. The administration of drug to systemic circulation via skin could be accomplished by utilization of transdermal patch that could sustain the drug release for proposed period of time in controlled manner. The drug is supplied at skin surface from where it diffuses across stratum corneum and reaches in systemic circulation through dermal capillaries.
Factors Controlling Absorption
The conventional delivery of drug through skin is a passive process and is ruled by Fick’s law, which states that flux or rate of absorption of a substance across a barrier is proportional to its concentration difference across that barrier. The maximum flux depends on saturation of drug in the vehicle (Cv) and the permeability coefficient Kp (equation 1). The permeability coefficient depends on properties of drug on barrier as well as in between the interaction of drug and barrier. The various factors imparting in this interaction are Partition coefficient (Km), Diffusion coefficient (D) and Length of diffusion pathway (L) (equation 2) [8,9]. Therefore, these four elements are responsible for control kinetics of drug absorption through skin.
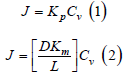
Role of the vehicle
The vehicle is an important link between drug potency and
therapeutic effectiveness, since extensive pharmaceutical research
has shown that the composition of the vehicle can profoundly
influence the rate and extent of absorption (bioavailability). As
illustrated by the potency ranking scale for glucocorticoids, the
same drug appears in different potency classes when formulated
in different vehicles. It was once axiomatic that ointments were
more potent than creams. Though true for the early glucocorticoid
products, it is no longer generally applicable [10]. Greater
understanding of the science underlying topical formulations
has allowed creams, gels, solutions and foams to be specifically
formulated equipotent to ointment. In the rational design of
dermatologic vehicles that maximize bioavailability, two factors are
of critical importance:
i. Solubilizing the drug in the vehicle (Cv); and
ii. Maximizing movement (partitioning) of drug from vehicle to
stratum corneum (Km). The partition coefficient describes the
ability of a drug to escape from the vehicle and move into the
outermost layer of the stratum corneum. It is defined as the equilibrium solubility of drug in the stratum corneum (sc)
relative to its solubility in the vehicle 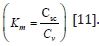
Drug concentration
The driving force for percutaneous absorption is the concentration of soluble drug in the vehicle. Many older topical drug products were marketed with the expectation that higher concentrations were more potent. Although true for some products, e.g. tretinoin gels and creams (0.01-0.1%) in which the drug is completely solubilized at all concentrations, for others it is not. Hydrocortisone 1% and 2.5% in a cream formulation have been shown to be of equal potency, as triamcinolone acetonide 0.025%, 0.1% and 0.5% creams. One of the major advances in formulating glucocorticoids, as first shown with fluocinonide, came when it was discovered that the addition of propylene glycol to the vehicle could completely solubilize the drug. This led to corticosteroid products with greater potency, as demonstrated in the vasoconstrictor assay [12]. Newer products are now tested during the development process to ensure that increased drug concentration results in increased bioavailability. However, excess non-dissolved drug can sometimes be advantageous; especially in transdermal patches worn for prolonged periods of time (e.g. up to a week). In this situation, as dissolved drug is absorbed into the body, non-dissolved drug can then become dissolved in order to maintain equilibrium, thereby maintaining a constant dissolved drug concentration over time and providing a constant rate of delivery [8].
Partition coefficient
In general, topically applied drugs are poorly absorbed because only a small fraction partitions into the stratum corneum. Most of the drug remains on the skin surface, subject to loss from a multitude of factors (exfoliation, sweating, wash-off, rub-off, adsorption onto clothing, and chemical or photochemical degradation). Even 10-12 hours following dosing, a drug that has not been lost by exfoliation or rub-off remains largely on the skin surface, and it is easily removed by a simple soap and water wash. In case the patches worn for several days, as much as half of the original amount of drug may still be present in the patch when it is removed, and this can pose a safety hazard upon disposal, especially with potentially dangerous drugs such as fentanyl [9]. A number of physical and chemical factors can improve partitioning. Hydration of the skin due to occlusion, either from a topical formulation or a patch, expands the reservoir volume available to drugs within the stratum corneum; this can increase absorption as much as five to tenfold. Common excipients such as ethanol and propylene glycol can also alter barrier structure so as to increase partitioning. In addition, many excipients have good solvent properties and, as a result, positively affect Cv as well as Km [10,11]. The use of high concentrations of propylene glycol to maximize bioavailability has become pervasive among the super and high potency corticosteroids, but at a price. Adverse events such as burning and stinging are common when such preparations are applied to fissured or eroded skin and contact dermatitis may occur. A number of other compounds have been identified as enhancers. Dimethyl sulfoxide (DMSO), the arche typical enhancer, exemplifies the effects that can be achieved. As with ethanol and propylene glycol, both Cv and Km are affected. Because DMSO is a superb solvent, higher drug concentrations can be achieved than with other solvents, but it also expands the stratum corneum barrier, permitting increased drug uptake and possibly an increased rate of diffusion (D) through the barrier. However, the use of powerful enhancers such as DMSO is constrained by excessive skin irritation or toxicity [13,14].
Regional variation
All body sites are not equally permeable. Variations in stratum corneum thickness, the number of sebaceous glands, and hydration status can all affect absorption. Current data and clinical experience suggest that one can crudely rank regional permeability as follows: nail << palm/sole < trunk/extremities < face/scalp << scrotum. [14,15].
Strategies to Enhance Transdermal Drug Delivery
Despite the significant permeability barrier of the stratum
corneum, drug delivery via the skin is a very attractive option
and is widely employed for both local and systemic therapy.
Topical treatment of cutaneous disorders obviously targets the
site of disease, thereby minimizing adverse side effects elsewhere
within the body. Delivery of systemic therapies via the skin avoids
degradation of the medication within the gastrointestinal tract and
first-pass metabolism by the liver, both of which are associated with
oral administration of drugs, in addition to evading the pain and
safety issues associated with injections. Transdermal delivery of
drugs, especially via long acting patches, enables infrequent dosing
and maintenance of steady state drug levels [16].
Many dermatologic medications can be applied topically to the
skin because the required dosage is often exceedingly small and
therefore they can be effective even in the setting of highly inefficient
absorption. In addition, a number of skin disorders are associated
with compromised barrier function, which leads to enhanced drug
uptake in sites of involvement. In contrast, systemic drug delivery
via the skin typically requires administration of larger doses
through normal skin. As a result, at the time of writing, only ~20
drugs have been FDA-approved for transdermal administration
[17]. The drugs contained within these patches share several
characteristics- they are low molecular weight (<400Da), lipophilic
(octanol–water partition coefficient up to 10000), and relatively
low dose (typically <10mg per day. Significant efforts have been
expended on the development of new approaches to enhance
transdermal drug delivery and thereby increase the number of
drugs administered via this route. These strategies can be broadly
subdivided into chemical, biochemical and physical approaches.
Chemical enhancement
Chemical enhancers include compounds that interact with the
lipid matrix of the stratum corneum to alter its nanostructure and
thereby increase permeability. The major advantages of chemical
enhancers are that they are typically low cost, can be incorporated
into a conventional patch or topical formulation, and do not require the complexity of a battery-powered device. The primary
disadvantage of chemical enhancers is that they are often associated
with skin irritation or toxicity when present at high concentrations
and with long exposure times [18]. Thus, chemical enhancers have
been employed principally to increase permeability to compounds
that already cross the skin reasonably well, but they have generally
been unable to significantly impact delivery of new classes of
molecules (e.g. highly water-soluble drugs) or macromolecules
such as proteins, gene-based medicines and vaccines [19]. The
most common chemical enhancer is water, which leads to hydration
of the stratum corneum when it accumulates during prolonged
occlusion; the occlusion can result from a topical formulation or a
patch. Following 24-48 hours of occlusion, corneocytes swell, the
intercellular spaces become distended, and the lacunar network
becomes dilated. Distention of lacunae is thought to eventually lead
to connections within an otherwise discontinuous system, creating
“pores” in the stratum corneum interstices through which polar
and non-polar substances can penetrate more readily [20].
Solvents, such as ethanol, methanol, chloroform and acetone,
as well as detergents can extract barrier lipids and/or disrupt their
bilayer structures, which then permeabilizes the stratum corneum.
Morphologic changes in human stratum corneum following
exposure on solvents include phase separation and disruption of
lamellar bilayers in addition to the creation of defects in corneocyte
membranes (with detergents). Moreover, surfactants, such as
sodium dodecyl (lauryl) sulfate, and vehicles (e.g. propylene glycol)
extract lipids and create extensive expansion of pre-existing lacunar
domains. Furthermore, solvent-based penetration enhancers, such
as azone, sulfoxides, urea and free fatty acids, not only extract
extracellular lipids, but they also alter stratum corneum lipid
organization (phase behavior), thereby increasing transdermal
delivery and expanding intercellular domains [21].
Biochemical enhancement
Biochemical methods have been developed to directly increase permeability of the stratum corneum lipid matrix as well as to indirectly affect skin permeability via alteration of lipid metabolism. Much of the work in this area has focused on peptides that are believed to disrupt or penetrate stratum corneum lipids. For example, polyarginine has been shown to ferry molecules attached to it across the stratum corneum and into the viable epidermis and dermis. Other peptides, identified by phage-display screening, appeared to target transfollicular pathways and did not require the drug to be attached. Magainin, a naturally occurring poreforming peptide, has been shown to increase skin permeability by direct interaction with and disruption of stratum corneum lipids [19,22]. In a related strategy, metabolically based approaches aim to enhance the efficacy of standard enhancers by biochemically inhibiting the repair (metabolic) response in vivo and there by delaying barrier recovery. This can be accomplished by altering the critical molar ratio of the three key stratum corneum lipids or by inducing discontinuities in the lamellar bilayer system. Both lipid synthesis inhibitors and agents that interfere with the assembly, secretion or extracellular processing of lamellar bodies have been examined, including brefeldin A, monensin, chloroquine, high Ca2+/ K+ levels and neutral pH buffers. Overall, biochemical enhancement methods are relatively new and to date they have not been used much in clinical drug delivery [23].
Physical enhancement
There are a number of physical methods to increase drug
delivery via the skin, many of which require the use of devices and
some of which hold the promise to significantly expand the spectrum
of drugs that can be administered transdermally to include watersoluble
molecules and macromolecules. Stripping is a simple
technique used in research protocols to remove stratum corneum
by sequential application of adhesive tape or cyanoacrylate glue.
Tape stripping removes both corneocytes and extracellular lipids,
thereby reducing the elongated path length that drugs otherwise
need to traverse, and it mechanically disrupts lamellar bilayers,
even in the retained lower stratum corneum layers. Barrier
disruption of human skin requires multiple strippings, which can
lead to inflammation. More strippings are required to disrupt the
barrier in skin phototypes V and VI (darkly pigmented) than in
phototypes I and II (lightly pigmented) subjects [24].
Iontophoresis and electroporation represent electrically
assisted, physical approaches to enhance delivery of drugs/
macromolecules across the stratum corneum. Iontophoresis uses
low currents applied for minutes to hours from an externally placed
electrode (with the same charge as the drug) in order to drive these
molecules across the stratum corneum, primarily by electrophoresis.
As the rate of drug delivery is generally proportional to the applied
current, iontophoresis offers an opportunity for programmable
drug delivery, especially with the recent development of
miniaturized microprocessor systems. Clinically, iontophoresis has
been employed to deliver: fentanyl and lidocaine for pain relief,
pilocarpine to induce sweating (as a diagnostic test) and tap water to
treat hyperhidrosis. Reverse iontophoresis has been used to extract
glucose from the skin as a means of monitoring glucose levels in
diabetic patients. Electroporation (electropermeabilization) utilizes
very short (microsecond to millisecond) and relatively high voltage
(~100V) electrical pulses to induce structural rearrangement
of stratum corneum lipids, leading to pore formation. Properly
designed systems can minimize sensations from the pulses and
facilitate delivery, especially of hydrophilic and charged molecules
into the skin. Although only at the research stage with regard to
transdermal delivery, electroporation is currently being used to
drive chemotherapeutic agents into superficial skin tumors by
applying surface or penetrating electrodes [25,26].
While ultrasound is widely and safely employed in both medical
diagnostics and physical therapy, this technology can also be used
to enhance transdermal delivery. When ultrasound is utilized in a
manner that resembles medical imaging, it is not very effective at
increasing skin permeability. However, ultrasound administered
in the context of heating deep tissues, for example during physical therapy, has been shown to increase drug penetration into the skin,
and this technique is actually used to increase local delivery of
anti-inflammatory agents at the time of physical therapy. With still
different settings (in particular low frequencies such as <1MHz),
ultrasound can be used to generate bubble activity, referred to as
“cavitation”. Cavitation bubbles oscillating and imploding in the
medium between the ultrasound transducer and the skin surface
generate shockwaves that mechanically impact the skin, creating
submicroscopic defects in stratum corneum structure. These
defects increase skin permeability to water-soluble molecules and
some macromolecules. In a related approach, pulsed laser beams
have also been used to generate photomechanical shockwaves at
the skin surface, which also increase skin permeability. Cavitational
ultrasound of the skin has been approved as a pretreatment
prior to the application of lidocaine as a means of accelerating
local anesthesia [8]. Now a days, nanomedicine has become
exceptionally critical as there has been a huge piece of novel
research and protecting develop in it. Nanoparticles for the delivery
of medications have acquired a lot of consideration in general
pharmacological properties of the usually utilized medication in
chemotherapy.
Basic Components of Transdermal Drug Delivery Systems
Polymer matrix or matrices
Polymers are employed in skin preparation and it strengthens the foundation of TDDS. Polymer selection and design are of prime importance in this system.
Considerations for polymer selection in transdermal delivery system
The polymer should be stable, non-reactive with the drug, easily manufactured and fabricated into desired product, and inexpensive. Properties of polymers (molecular weight and glass transition temperature and chemical functionality) should be such that the specific drug diffuses properly and get released through it and mechanical characteristics of the polymer should not deteriorate excessively when large amount of active agents are incorporated into it, should have biocompatibility and chemical compatibility with the drug and other components of the system such as penetration enhancers and PSAs, should provide consistent and effective delivery of a drug throughout the product’s life. Polymers are utilized in TDDS in versatile manner including as: rate-controlling membranes, adhesives (pressure-sensitive adhesives), backing layers, and release liner [27].
Rate controlling membrane
The elementary way to control the release of a drug is to disperse through an inert polymeric matrix. In this system, the drug is physically blended with polymeric powder (either hydrophilic or lipophilic), and the medicated polymer is then molded into a medicated disc with a defined surface area and controlled thickness. An inverse relationship is thus observed between the release rate and membrane thickness. Moreover such relationship was confirmed when occurrence of boundary layer effects in permeability measurements. Permeation rate (J) of the membranes which was synthesized from three different monomers of A, B and C (2-hydroxy-3-phenoxypropylacrylate, 4-hydroxybutyl acrylates, sec-butyl tiglate respectively) at different membrane thickness was determined (L), using Clonidine as test drug by applying expression of Fick’s law of diffusion. Release rates of the drug are improved by the addition of a hydrophilic polymer, e.g. hydroxypropyl methylcellulose, to the rate-controlling membrane. Drug transfer from the hydrophilic matrix across the membrane is shown to be controlled by the drug partitioning from the matrix into the membrane. Thus, the diffusion properties of the membrane are used to ensure availability of the drug and/or excipients to the skin [28].
Adhesives
The adhesive, a vital component plays an intimate contact
between the delivery system with the skin. The adhesion of TDDS
is one of the critical factors to the safety, efficacy and quality of
the product. It is related to drug delivery and therapeutic effect.
It carries the drug which can either be dispersed or dissolved
in the matrix or the compartment containing drug (solution or
suspension) is separated from the adhesive layer by a diffusion
controlling membrane, the drug permeates through this adhesive
membrane to reach the skin. Quality of bond between patch and
skin holds importance as it directly reflects consistency of drug
delivered. The delivery of drug from the patch diminishes as a
result of patch lift, or falling off, reduces surface area of contact. In
other words, poor adhesion results in improper dosing of patients.
Secondly, patches that fail to adhere for their prescribed time phase
must be replaced more frequently, thereby increasing the patient’s
cost. Thirdly, lack of adhesion is a safety issue. There are potential
hazards when accidentally exposed (e.g. transfer of a patch from
an adult to a child while hugging, accidentally sitting or lying on a
patch [29].
Pressure Sensitive Adhesives (PSA): The transdermal devices
to the skin can be done by using a pressure sensitive adhesive
which can be positioned on the face of the device or in the back of
the device and extending peripherally. The three most commonly
used adhesives are polyisobutylene, polyacrylate and silicones
in TDD devices. Natural rubber karaya gum-based adhesives are
another class of PSAs used in many Over The Counter (OTC) dermal
therapeutic systems [30].
Newer inventions in the field of PSA: The first approach
involves the development of new polymers, which include hydrogel
hydrophilic polymers, and polyurethanes. The second approach
is to physically or chemically modify the chemistries of the PSAs
in current use (such as PIBs, silicones, and acrylates). Physical
modification refers to the formulation of the base adhesives with
some unique additives so that, in synergy with the drug and
excipients in the system formulation, the result is enhanced drug delivery and improved skin-adhesion properties [31]. Chemical
modification involves chemically incorporating or grafting
functional monomers to the conventional PSA polymers in order to
improve drug delivery rates.
Release liners
During storage the patch is covered by a protective liner that is removed and discarded before the application of the patch to the skin. Since the liner is in intimate contact with the TDDS, the liner should be chemically inert. The release liner is composed of a base layer which may be non-occlusive (e.g. paper fabric) or occlusive (e.g. polyethylene, polyvinylchloride) and a release coating layer made up of silicon or teflon. Other materials used for TDDS release liner include polyester foil and metalized laminate [32].
Backings
Backings are chosen for appearance, flexibility and need for occlusion. Examples of backings are polyester film, polyethylene film and polyolefin film, and aluminum vapor coated layer. Other assiduities are the backing additives leaching out and diffusion of drug or the compositions, through the backing. An overemphasis on the chemical resistance often may lead to stiffness and high occlusivity to moisture vapor and air. It causes the TDDS to lift and may possibly irritate the skin during long-term use [33].
Drug
Transdermal delivery of drugs has taken a surge of popularity nowadays. Various physicochemical, pharmacokinetic and pharmacological properties of the drug are considered for TDS development. Because of the limited permeability of the skin, drugs have to be transdermally delivered by passive diffusion through the skin and are limited by several substantial constraints. Transdermal delivery is limited to drugs used in low doses. For the drug molecule having a small molecular weight of 1000 Da, adequate solubility in the vehicle, log P value, melting point of 200°C and appropriate lipophilicity are considered as suitable candidates for delivery via this route [34].
Penetration enhancers
These are compounds which promote skin permeability by altering the skin as a barrier to the flux of a desired penetrant. Penetration enhancers are incorporated into a formulation to improve the diffusivity and solubility of drugs through the skin that would reversibly reduce the barrier resistance of the skin. Thus allow the drug to penetrate to the viable tissues and enter the systemic circulation [35].
Desirable properties for penetration enhancers: Desirable properties for penetration enhancers acting within the skin should be non-irritant, non-sensitizing, non-phototoxic, and noncomedogenic; ideally work rapidly, and the activity and duration of effect should be both predictable and reproducible; have no pharmacological activity within the body- i.e., should not bind to receptor sites; work unidirectional, i.e., should allow therapeutic agents into the body while preventing the loss of endogenous material from the body; shows barrier properties which must return both rapidly and fully when removed from the skin; show compatibility with formulation and system components; be odorless, tasteless, colorless, and cosmetically acceptable; have a desired solubility parameter that approximates that of the skin [36].
Influence of penetration enhancers on the structure of the
SC: Diffusional resistance known to reside in the SC is embodied
by a complex interaction of lipid and proteinaceous components
in which fairly distinct hydrophilic and lipophilic penetration
pathways are created. The make-up and function of the SC in
recent years have resulted in a diverse range of enhancers being
tested for their ability to facilitate improved permeation of the skin
portal by co-administered drugs [37]. The biochemical order of the
intercellular lipid matrices of the SC or the keratinized environment
of the corneocytes is altered to allow the penetration of compounds
at a suitable rate to the desired site of activity. Barry worked on the
possible interactions between penetration enhancers and the SC and
put forward the Lipid-Protein Partitioning (LPP) theory. According
to this theory, the three main mechanisms of enhancement are
i. Interactions with the intercellular lipids;
ii. Interactions with the intracellular keratin and
iii. The penetration of high amounts of enhancers or so-called
co-solvents into the SC with a resulting improved dissolving
capacity of the barrier for drugs and/or co-enhancers. Some
of the examples of the widely used classical enhancers involve
various classes that include water, hydrocarbons alcohols,
acids amines, amides, esters, surfactant terpenes, terpenoids
and essential oil, sulfoxides, lipids and miscellaneous such as
cyclodextrin derivatives, chitosan [38,39].
Plasticizers
Plasticizers have also been used in many formulations ranging from 5 to 20% (w/w, dry basis). Along with the brittleness and ductility of the film, it is also responsible for adhesiveness of the film with other surfaces or membranes and improvement in strength of film. Some of its examples are glycerol or sorbitol, at 15%,w/w, dry basis, phthalate esters, phosphate, esters, fatty acid esters and glycol derivatives such as PEG 200, and PEG 400. The selection of an appropriate plasticizer and its concentration has a profound influence on the mechanical properties as well as on the permeability of drugs [38,40].
Conclusion
In recent Scenario, Transdermal Drug Delivery has revolutionized over Oral Drug Delivery system. But, major challenge in fabricating a patch needs the insights about governing factors of drug along with the excipients. The review aimed current knowledge necessary for fabrication of novel transdermal patch.
References
- Agrawal S, Pruthi J (2011) Development and evaluation of matrix type transdermal patch of ethinylestradiol and medroxyprogesterone acetate for anti-implantation activity in female Wistar rats. Contraception 84(5): 533-538.
- Ah Y, Choi J, Choi Y, Ki H, Bae J (2010) A novel transdermal patch incorporating meloxicam: In vitro and in vivo Int J Pharm 385(1-2): 12-19.
- Ahad M, Kohli K, Sultana Y, Mujeeb M, Ali A, et al. (2010) Transdermal drug delivery: The inherent challenges and technological advancements. Asian J Pharm Sci 5(6): 276-288.
- Ajazuddin, Alexander A, Amarji B, Parijat K (2012) Synthesis characterization and in vitro studies of pegylated melphalan conjugates. Drug Dev Ind Pharm 39(7): 1053-1062.
- Babu RJ, Pandit JK (2005) Effect of penetration enhancers on the release and skin permeation of bupranolol from reservoir-type transdermal delivery systems. Int J Pharm 288(2): 325-334.
- Candi E, Schmidt R, Melino G (2005) The cornified envelope: A model of cell death in the skin. Nat Rev Mol Cell Biol, pp. 328-340.
- Chein Y (1987) Transdermal controlled systemic medications. In: Marcel D (ed.), (2nd edn), USA, pp. 1-500.
- Karwoski A, Plaut R (2004) Experiments on peeling adhesive tapes from human forearms. Skin Res Technol 10(4): 271-277.
- Lipinski CA, Lombardo F, Dominy WB, Feeney JP (2001) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 46(1-3): 3-26.
- Lopez FR, Seto EJ, Blankschtein D, Langer R (2011) Enhancing the transdermal delivery of rigid nanoparticles using the simultaneous application of ultrasound and sodium lauryl sulfate. Biomaterials 32(3): 933-941.
- Lopez-Cervantes M, Escobar-Chavez J, Casas-Alancaster N, Turlier V, Lauze C (2009) Development and characterization of a transdermal patch and an emulgel containing kanamycin intended to be used in the treatment of mycetoma caused by Actinomadura madurai. Drug Dev Ind Pharm 35(12): 1511-1521.
- Kai T, Mak W, Potts R, Guy HR (1990) Mechanism of percutaneous penetration enhancement: Effect of n-alkanols on the permeability barrier of hairless mouse skin J Control Release 12: 103-112.
- Naik A, Kalia NY, Guy HR (2000) Transdermal drug delivery: Overcoming the skin's barrier function. Pharm Sci Technol Today 3(9): 318-326.
- Naruse M, Ogawara K, Kimura T, Konishi R, Higaki K (2012) Development of transdermal therapeutic formulation of CNS5161, a novel N-methyl-D-aspartate receptor antagonist, by utilizing pressure-sensitive adhesives I. Biol Pharm Bull 35(3): 321-328.
- Nesseem ID, Eid SF, El-Houseny SS (2011) Development of novel transdermal self-adhesive films for tenoxicam, an anti-inflammatory drug. Life Sci 89(13-14): 430-438.
- Panchaxari M, Pampana S, Pal T, Devabhaktuni B, Aravapalli AK (2013) Design and characterization of diclofenac diethylamine transdermal patch using silicone and acrylic adhesives combination. Daru 21(1): 1-6.
- Patel K, Patel H, Patel V (2012) Formulation and characterization of drug in adhesive transdermal patches of diclofenac acid. Int J Pharmacy Pharm Sci 4(1): 296-299.
- Prausnitz RM, Mitragotri S, Langer R (2004) Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov 3(2): 115-124.
- Lal N, Yadav P, Rastogi V, Verma A, Verma N (2017) Aspects of pressure sensitive adhesives in fabricating drug-in-adhesive transdermal therapeutic systems. Drug Delivery Letter 7(1): 3-15.
- Lal N, Yadav P, Rastogi V, Pandey L, Verma N, et al. (2016) Development and evaluation of transdermal therapeutic system of metoprolol succinate using acrylic polymer. Asian J Pharm 10(3): 178-187.
- Raynaud PJ, Auges M, Liorzou L, Turlier V, Lauze C (2009) Adhesiveness of a new testosterone-in-adhesive matrix patch after extreme conditions. Int J Pharm 375(1-2): 28-32.
- Niharika, Navneet V (2016) Adhesive polymers in fabrication of transdermal drug delivery. Research J Pharm and Tech 9(7): 945-956.
- Ren C, Fang L, Ling L, Wang Q, Liu S (2009) Design and Invivo evaluation of an indapamide transdermal patch. Int J Pharm 370(1-2): 129-135.
- Savoji H, Mehdizadeh A, Abadi ARS (2014) Transdermal nitroglycerin delivery using acrylic matrices: Design, formulation, and in vitro SRN Pharm, p. 1-9.
- Taghizadeh M, Soroushnia A, Mohamadnia F (2010) Preparation and in vitro evaluation of a new fentanyl patch based on functional and non-functional pressure sensitive adhesives. AAPS Pharm Sci Tech 11(1): 278-284.
- Valenta C, Auner GB (2004) The use of polymers for dermal and transdermal delivery. Eur J Pharm Biopharm 58(2): 279-289.
- Wang E, Casciano CN, Clement RP, Johnson WW (2001) Active transport of fluorescent P-glycoprotein substrates: Evaluation as markers and interaction with inhibitors. Biochem Biophys Res Commun 289(2): 580-585.
- Furuishi T, Fukami T, Suzuki T, Tomono K, Io T (2008) Formulation and in vitro evaluation of pentazocine transdermal delivery system. Biol Pharm Bull 31(7): 1439-1443.
- Ren C, Fang L, Ling L, Wang Q, Liu S, et al. (2009) Design and in vivo evaluation of an indapamide transdermal patch. Int J Med Pharm 370(1-2): 129-135.
- Chowhan ZT, Pritchard R (1978) Effect of surfactants on percutaneous absorption of naproxen I: comparisons of rabbit, rat, and human excised skin. J Pharm Sci 67(9): 1272-1274.
- Cooper RE, Merritt WE, Smith LR (1985) Effect of fatty acids and alcohols on the penetration of acyclovir across human skin in vitro. J Pharm Sci 74(6): 688-689.
- Bolzinger M, Briancon S, Pelletier J, Chevalier Y (2012) Penetration of drugs through skin, a complex rate-controlling membrane. Curr Opin Colloid Interface Sci 17(3): 156-165.
- Waters C (2013) The development of the rotigotine transdermal patch: A historical perspective. Neurol Clin 31(3): S37-S50.
- Vora N, Madan PL, Lin S (2013) Development and in-vitro evaluation of an optimized carvedilol transdermal therapeutic system using experimental design approach. Asian J Pharm 8(1): 28-38.
- Toddywala R, Ulman K, Walters P, Chien WY (1991) Effect of physicochemical properties of adhesive on the release, skin permeation and adhesiveness of adhesive type transdermal drug delivery systems (a-TDD) containing silicone-based pressure sensitive adhesives. Int J Pharm76(1-2): 77-89.
- Taghizadeh SM, Soroushnia A, Mirzadeh H, Barikani M (2009) Preparation and in vitro evaluation of a new fentanyl patch based on acrylic/silicone pressure-sensitive adhesive blends. Drug Dev Ind Pharm 35(4): 487-498.
- Sun Y, Fang L, Zhu M, Li W, Meng P, et al. (2009) A drug-in-adhesive transdermal patch for S-amlodipine free base: In vitro and in vivo Int J Pharm 382(1-2): 165-171.
- Smolinske SC (1992) Handbook of food, drug and cosmetic excipients. (1st edn), CRC Press, USA, pp. 1-448.
- Roy SD, Flynn GL, Gutierrez M, Cleary GW (1996) Characterizations of pressure-sensitive adhesives for matrix patch design. J Pharm Sci 85(5): 491-495.
- Santoyo S, Arellano A, Ygartua P, Martin C (1995) Penetration enhancer effects on the in vitro percutaneous absorption of piroxicam through rat skin. Int J Pharm 117(2): 219-224.
© 2021 Niharika Lal. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






