- Submissions

Full Text
Determinations in Nanomedicine & Nanotechnology
Applications of Nanotechnology in Wastewater Treatments
Alka, Laura JS and Dhania G*
Department of Environmental Sciences, India
*Corresponding author: Geeta Dhania, Department of Environmental Sciences, India
Submission: August 09, 2021;Published: September 27, 2021

ISSN: 2832-4439 Volume2 Issue2
Abstract
Recent innovations in nanotechnology have transformed a number of scientific and industrial areas including the wastewater treatment. Nanoparticles or nanomaterials are considered to be most significant and appropriate for the treatment and purification of waste as they are highly reactive, have high degree of functionalization, large surface area to volume ratio and have a good affinity for target substances and their extremely small size makes them most appropriate purification agents. Nanotechnology has led to various efficient ways of treating the wastewater in a more accurate and precise manner on both small and large scale. This article mainly reviews the recent advances of nanotechnology and its application in the field of wastewater treatment.
Keyword: Nanofiltration; Nanoparticles; Nanomaterials; Nanotechnology; Nanosorbents; Wastewater treatment
Introduction
Over the years, nanotechnology has brought revolutions in many sectors. Both developing and developed countries are growing interest in investing more in this field [1]. Nanotechnology is a wide area, it adopts the characteristics of various disciplines like chemistry, physics and biology. It involves the creation, measurements, utilization and manipulation of materials and systems at a length of the nanometer sizes, usually from scale of 1 to 100nm [2,3]. Dealing with this level of small sizes, these materials are loaded with the most important feature of having high area to volume ratio that makes the uses of these nanomaterials in multidisciplinary fields [4]. Nanotechnology offers a wide range of applications in areas like life science, environment, agriculture, food, and medicine, etc. Nanoparticles have dimensions comparable with that of viruses, this permits them to attach themselves with various biological entities without altering their functions. Their large surface area allows them to make a strong bond with surfactant molecules, this led to detection of some specific polluting contaminants, interaction with them and their treatment [5]. Nanoparticles also have technological and fundamental interest as they provide a good alternative solution to many technological problems and environmental challenges in the fields of wastewater treatment, solar energy conversion, medicine, and catalysis. It is also gaining interest in healthcare, drug- gene delivery, energy science, optics, mechanics, food and feed, space industries, chemical industries, electron transmitters, light emitters, and cosmetics [6]. Materials having the dimensions of nanoscale shows a great difference in the characteristics and properties when compared to the same material in bulk. This difference in the physical and chemical properties of the atoms, molecules and their bulk counterparts arises due to different physiochemical properties and surface to volume ratio.
Nanotechnology and Wastewater Treatment
Nanotechnology is the manipulation, control, and integration of atoms and molecules at the nanoscale to create materials, structures, components, devices, and systems. The development of various tools and techniques enabled by nanotechnology, especially in the area of water purification, opens up a new potential alternative to improve the efficiency and cost-effectiveness of wastewater treatment [7]. This is conceivable because nanomaterials are small, highly reactive, more precise, and, most crucially, they can be manufactured using environmentally benign and potentially cost-effective procedures [8]. Nano catalysis, Nanofiltration, and Nano sorbents are some of the potential water treatment techniques/tools offered by nanotechnology.
Nano catalysts
Nano catalysts are also commonly employed in water
treatment because they boost catalytic activity at the surface
due to their unique traits of having a larger surface area and
shape-dependent properties. It improves pollutant reactivity and
degradation. For the degradation of environmental contaminants
such as polychlorinated biphenyls, azo dyes, halogenated aliphatic,
organochlorine pesticides, halogenated herbicides, and nitro
aromatics, semiconductor materials, zero-valence metal, and
bimetallic nanoparticles are commonly used catalytic nanoparticles
[9]. On a laboratory scale, the catalytic activity has been
demonstrated for a variety of pollutants. Because redox reactions
are employed to make active catalysts on a large scale, there is a
need to reduce hydrogen use and maintain hydrogen economy by
manufacturing catalysts directly in metallic form.
Several studies have been conducted on the immobilization of
metallic nanoparticles in membranes (such as cellulose acetate,
Polyvinylidene Fluoride (PVDF), polysulfone, chitosan, and others)
for effective degradation and dechlorination of toxic contaminants,
which offers several advantages such as high reactivity, organic
partitioning, nanoparticle prevention, lack of agglomeration, and
reduced surface [10].
Nanofiltration
Filtration, which incorporates a filter media or a membrane that separates the solid from the liquid, is one of the most common and significant procedures in water purification and wastewater treatment. Figure 1 depicts the various membrane-based filtering processes, as well as the sizes and types of particles that can be filtered out.
Figure 1: Membrane based filtration techniques with effective size and types of particles.
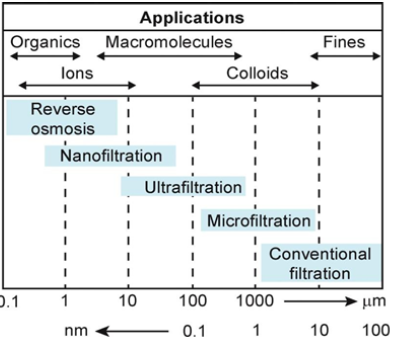
Nanofiltration is a pressure-driven membrane separation
technique and is rapidly advancing in the area of water purification
and wastewater treatment due to its unique charge-based repulsion
property and high rate of permeation. Due to the lower pressure
requirements (7-30atm) compared to reverse osmosis processes
(20-100atm), nanofiltration is becoming more popular these days,
being a lower energy consumption technique [11].
Several studies have been conducted on the immobilization of
metallic nanoparticles in membranes (such as cellulose acetate,
polyvinylidene fluoride, polysulfone, chitosan, and others) for
effective degradation and dechlorination of toxic contaminants,
which offers several advantages such as high reactivity, organic
partitioning, nanoparticle prevention, lack of agglomeration, and
reduced surface [10].
Nanocomposites films made of polyetherimide, and palladium
acetate have been examined for particular interactions between
hydrogen and palladium-based nanoparticles, demonstrating their
efficacy in water treatment. By annealing the precursor film under
various circumstances, both in situ and ex situ, metal nanoparticles
were formed within the matrix. This opens up the possibility of
creating materials with customizable qualities [12].
Nano sorbents
Sorption is the process by which one material, sorbate, binds
to another substance, sorbent, through physical or chemical
interactions. Sorbents are a type of separation media used in
water purification and treatment to remove organic and inorganic
pollutants from contaminated water. In general, there are three processes to the sorption of contaminants in water on the sorbent
surface:
a) Pollutant transport from the water to the sorbent surface,
b) Sorbent surface adsorption, and
c) Transit inside the sorbent. Nanoparticles have two key
characteristics that make them excellent sorbents.
Gram-positive bacteria, Gram-negative bacteria, and bacterial
spores are all resistant to magnesium oxide nanoparticles and
cellulose acetate fibers with embedded silver nanoparticles [13].
Kuo et al. [14] studied the adsorption of organic dyes from
water using carbon nanotubes was investigated, and it was
discovered that dye adsorption on the carbon nanotube surface
is driven by a physisorption mechanism. Carbon nanotubes and
activated carbon have a high adsorption rate and capacity, and
both are thermally and chemically stable materials, making them
appropriate for water treatment. Due to their small size, full
separation of carbon nanotubes and powdered activated carbons
from water is challenging. Integration of magnetic nanoparticles
with carbon nanotubes and activated carbon has been found to be
particularly effective in addressing this issue [15].
Tino et al. [16] used a mini-emulsion polymerization technique
to create molecularly imprinted nanospheres for the specific
adsorption of micro pollutants from hospital wastewater effluents.
This technique is very complex but can be done in a single reaction
chamber, resulting in particles with sizes ranging from 50nm
to 500nm. A magnetic core can be added to allow for the final
separation of these nanospheres, as well as the more important
known harmful pollutants, from wastewater.
Conclusion
The organic and inorganic wastes in water can be controlled using current wastewater treatment procedures. However, because of the inability to properly purify water and the impossibility to reuse the retentates, these technologies are energy intensive and uneconomical. Nanomaterials-based processes are more costeffective, use less time and energy, and produce far less waste than bulk materials-based approaches. However, specific safeguards must be taken to ensure that nanoparticles do not pose a hazard to human health or the environment. Nanotechnology has the potential to have a significant impact on wastewater treatment in the future. Nanotechnology aims to improve existing methods by increasing process efficiency and increasing the reusability of nanomaterials, lowering the plant or process’s operating costs. Nanomaterials have unique qualities such as a high surface-tovolume ratio, high reactivity and sensitivity, the ability to selfassemble on substrates to form films, high adsorption, and so on, making them ideal for water treatment. As further progress is made in the field of nanomaterials, they will become a key component of industrial and wastewater treatment systems in the future.
References
- Qureshi AM, Swaminathan K, Karthikeyan P, Ahmed K, Sudhir P, et al. (2012) Application of nanotechnology in food and dairy processing: An overview. Pak J Food Sci 22: 23-31.
- Schmid G (2010) Nanoparticles: From theory to application 2nd (edn), p. 533.
- Dixon MB, Falconet C, Ho L, Chow CW, O’Neill BK, et al. (2011) Removal of cyanobacterial metabolites by nanofiltration from two treated waters. Journal of Hazardous Materials 188(1-3): 288-295.
- Sharma V, Sharma J (2017) Electron microscopy study of green synthesized zero valent iron nanoparticles. Int J Eng Technol Sci Res 4(5): 654-658.
- Nguyen HL, Nguyen HN, Nguyen HH, Luu MQ, Nguyen MH (2014) Nanoparticles: Synthesis and applications in life science and environmental technology. Advances in Natural Sciences: Nanoscience and Nanotechnology 6(1): 015008.
- Ahmad N, Sharma S, Singh VN, Shamsi SF, Fatma A, et al. (2011) Biosynthesis of silver nanoparticles from Desmodium triflorum: A novel approach towards weed utilization. Biotechnology Research International, pp. 1-8.
- Hornyak GL, Moore JJ, Tibbals HF, Dutta J (2009) Fundamentals of nanotechnology, CRC Press, Taylor and Francis Group, New York, USA.
- Peng X, Luan Z, Di Z, Zhang Z, Zhu C (2005) Carbon nanotubes-iron oxides magnetic composites as adsorbent for removal of Pb(II) and Cu(II) from water. Carbon 43: 880-883.
- Zhao X, Lv L, Pan B, Zhang W, Zhang S, et al. (2011) Polymer-supported nanocomposites for environmental application: A review. Chemical Engineering Journal 170( 2-3): 381-394.
- Xu J, Bachas L, Bhattacharyya D (2009) Synthesis of nanostructured bimetallic particles in poly ligand functionalized membranes for remediation applications. Nanotechnology Applications for Clean Water, pp. 311-335.
- Drewes JE, Bellona CL, Xu P (2008) Comparing nanofiltration and reverse osmosis for treating recycled water. IWA Publishing (International Water Assoc), London, UK.
- Clémenson S, Espuche E, David L, Léonard L (2010) Nanocomposite membranes of polyetherimide nanostructured with palladium particles: Processing route, morphology and functional properties. Journal of Membrane Science 361(1-2): 167-175.
- Savage N, Diallo MS (2005) Nanomaterials and water purification: Opportunities and challenges. Journal of Nanoparticle Research 7: 331-342.
- Kuo CY, Wu CH, Wu JY (2008) Adsorption of direct dyes from aqueous solutions by carbon nanotubes: Determination of equilibrium, kinetics and thermodynamics parameters. J Colloid Interface Sci 327(2): 308-315.
- Gorria P, Sevilla M, Blanco JA, Fuertes AB (2006) Synthesis of magnetically separable adsorbents through the incorporation of protected nickel nanoparticles in an activated carbon. Carbon 44(10): 1954-1957.
- Schreibera T, Webera A, Niedergalla K, Rieglera J, Brynioka D, et al. (2009) Water treatment by molecularly imprinted polymer nanoparticles. MRS Spring Meeting. Cambridge Journals Online 11(69).
© 2021 Dhania G. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






