- Submissions

Full Text
Determinations in Nanomedicine & Nanotechnology
Porous Anodic Alumina for Some Photonic Devices
Mukhurov N*, Gasenkova I and Zhvavyi S
State Research and Production Association, Belarus
*Corresponding author: Mukhurov N, State Research and Production Association, Belarus
Submission: March 16, 2020;Published: September 30, 2020

ISSN: 2832-4439 Volume2 Issue1
Abstract
The photoluminescent properties of the porous anodic alumina and some of its practical applications for photonic devices are briefly considered.
Keyword: Anodic Alumina; Optical Properties; Photoluminescence; Photonic devices
Introduction
The scientific and technological interest in porous anodic alumina (PAA) is attributable to its numerous applications. A well-known application of PAA is its use as a base material for creating photonic crystals [1, 2]. Depending on the anoding conditions (concentration and type of electrolyte, applied potential, temperature and time of anodizing), highly ordered nanoporous oxide structures with pore diameter from 10 to 300nm and interpore distance from 35 to 500nm can be obtained. In addition, a number of electrochemical approaches have been proposed for the formation of PAA with complex pore geometries [3-5], which can significantly expand the possibilities for the formation of photonic crystals and their applications [6].
Photoluminescent Properties of PАА
Figure 1: The PL spectra of the original PAA samples (1) and samples annealed in air at Ta = 200 °C (2), 400 °C (3) and 600 °C (4), obtained at excitation wavelength λex = 275nm [17].
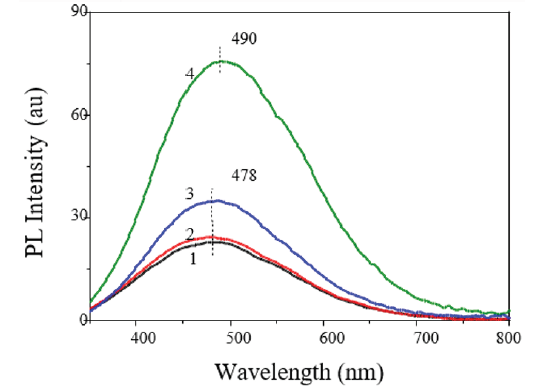
Considerable attention is given to the study of optical properties of PAA because they certainly have an impact on the parameters of photonic and optoelectronic structures. PAA has a high transmittance (~80-90%) in the visible and infrared parts of the spectrum, and high absorption in the UV range. Under the influence of UV radiation on the PAA at <350nm, a broad photoluminescence (PL) band is observed in the 350-650nm range with a maximum at 450-500nm (Figure 1). The shape of the spectrum and the PAA PL intensity depend on the conditions of the electrochemical process and subsequent heat treatment. It was found that the PAA formed in an oxalic acid electrolyte had the highest PL intensity compared to that formed in electrolytes based on sulfuric, phosphoric or malonic acids [7]. Besides, during heat treatment of PAA, an increase in the PL intensity is observed with the rise of the annealing temperature Та from 300 оС to 600 °С [8-11]. As the annealing temperature is further increased, the PAA PL intensity falls sharply (Figure 2). For annealing in vacuum to 600 °С, the increase in the PAA PL intensity is much larger than for annealing in air [12]. Depending on the goals and objectives of optical sensors developed using PAA, the PL properties can be enhanced or weakened by an appropriate choice of anodizing parameters and subsequent thermal annealing.
Figure 2: The dependence of the ratio of the integrated values of the PL intensities to the corresponding values of the original samples annealed in vacuum (1,2) and in air (3,4) on the annealing temperatures Ta for excitation wavelengths λex = 275 (1,3) and 325 (2,4)nm.

The Use of PAA in Development of Photonic Devices
Due to their physico-chemical properties, PAA structures hold promises in the area of development of optically active devices. Many studies have demonstrated the use of anodic alumina for making optical filters, antireflection surfaces, resonators, or micro resonators [6]. The PL stability, high sensitivity to changes in the effective optical length, and biocompatibility make it possible to use PAA to develop optical sensors for biology and medical applications. The spectral stability of the PAA photoluminescence can be employed to create optical sensors with high resolution, sensitivity, and biocompatibility. For the first time, it was shown in [13] that the presence of adsorbed molecules of morin and morintrypsin in PAA can be detected by shifts in the PL spectrum.
The works [14,15] have revealed that for certain geometric parameters (porosity and thickness) of the PAA films the PL spectrum shows interference fringes due to PL enhancement at the wavelengths corresponding to the optical modes of the Fabry- Perot resonator formed by the air/PAA/Al system. By using these properties of PAA, the authors of [16] have devised an optical barcode system for optical biosensors based on the PAA PL spectrum in the UV-visible region. The source of these PL oscillations is the Fabry- Petro effect which enhances the PL fluctuations. In this system, each barcode corresponds to a fluctuation in the PL spectrum. The number, intensity and position of these oscillations can be adjusted by changing the length and diameter of the pores.
PAA has found a successful use in the development of SERS substrates [17-19] that are formed by sputtering gold or silver on the upper or lower surface of PAA substrates. Such SERS-PAA platforms can be designed over a wide range of desired geometrical parameters by choosing anodizing and metal deposition conditions to obtain optimized SERS signals for specific applications. It has been demonstrated that SERS-PAA platforms with gold or silver nanoparticles on their surface provide the compound signal amplification of up to 106. The paper [20] has studied an achromatic phase plate based on a PAA membrane 10μm thick with a periodic system of nanoholes parallel to each other over the entire area and perpendicular to both surfaces with a diameter of 60-80nm and an interpore distance of 100-120nm.
It is shown that such a phase plate ensures a variable phase shift of the orthogonally polarized components of radiation passing through the plate and can function as a quarter-wave and halfwave plates in the spectral range from 400 to 1000nm. In this case, the transition from one mode to another is affected by varying the orientation of the film relative to the incident light beam. Regardless of the progress made in the study of the PAA properties and devices based on it, further research is needed into the methods of formation and modification of the oxide to develop a wide range of photonic structures.
References
- Takayama O, Cada M (2004) Two-dimensional metallo-dielectric photonic crystals embedded in anodic porous alumina for optical wavelengths. Applied Physical Letters 85(8): 1311.
- Santos A (2017) Nanoporous anodic alumina photonic crystals: fundamentals, developments and perspectives. J Materials Chemistry C 5(23): 5581-5599.
- Lee W, Roland S, Ulrich G (2008) A continuous process for structurally well-defined Al2O3 nanotubes based on pulse anodization of aluminum. Nano Letters 8(8): 2155-2160.
- Santos A, Pilar F, Josep P, Josep F, Lluís F, et al. (2011) Structural engineering of nanoporous anodic alumina funnels with high aspect ratio. Journal of Electroanalytical Chemistry 655(1): 73-75.
- Sulka GD, Agnieszka B, Lifeng L (2011) Fabrication of diameter-modulated and ultrathin porous nanowires in anodic aluminum oxide templates. Electrochimica Acta 56: 4972-4979.
- Law CS, Siew YL, Andrew DA, Nicolas HV, Abel S, et al. (2018) nano porous anodic alumina photonic crystals for optical chemo- and biosensing: Fundamentals, advances, and perspectives. Nanomaterials 8(10): 788.
- Mukhurov NI, SP Zhvavyi, SN Terekhov, AY Panarin, IF Kotova, et al. (2008) Influence of electrolyte composition on photoluminescent properties of anodic aluminum oxide. Journal of Applied Spectroscopy 75: 214-218.
- Stojadinovic S, Z Nedic, I Belca, RVasilic, B Kasalica, et al. (2009) The effect of annealing on the photoluminescent and optical properties of porous anodic alumina films formed in sulfamic acid. Applied Surface Science 256(3): 763-767.
- Sun X, Faqiang X, Zongmu L, WenhuaZ (2006) Photoluminescence properties of anodic alumina membranes with ordered nanopore arrays. Journal of Luminescence 121(2): 588-594.
- Ilin DO, Vokhmintsev AS, IA Weinstein (2016) Luminescence characteristics of nano porous anodic alumina annealed at different temperatures. AIP Conference Proceeding 1767(1): 10.
- Gasenkova IV, NI Mukhurov, SP Zhvavyi, EE Kolesnik, АP Stupak, et al. (2017) Photoluminescent properties of nano porous anodic alumina doped with manganese ions. Luminescence. 185: 298-305.
- IV Gasenkova, NI Mukhurov, SP Zhvavyi, EE Kolesnik, АP Stupak, et al. (2019) Effect of heat treatment in vacuum on photoluminescence of anodic alumina. Luminescence 34(5): 520-525.
- Jia RP, Y Shen, HQ Luo, XG Chen, ZD Hu, et al. (2004) Enhanced photoluminescence properties of morin and trypsin absorbed on porous alumina films with ordered pores array. Solid State Communications 130(6): 367-372.
- K Huang, L Pu, Y Shia, P Han, R Zhang, et al. (2006) Photoluminescence oscillations in porous alumina films. Applied Physical Letters 89(20): 10.
- Gardelis S, AG Nassiopoulou, V Gianneta, M Theodoropoulou (2010) Photoluminescence-induced oscillations in porous anodic aluminum oxide films grown on Si: Effect of the interface and porosity. Journal of Applied Physics 107(11): 10.
- A Santos, VS Balderrama, M Alba, P Formentín, J Ferré‐Borrull, et al. (2012) Nanoporous anodic alumina barcodes: Toward smart optical biosensors. Advanced Materials 24(8): 1050-1054.
- Seung JL, Zhiqiang G, Hongxing X, Martin M (2007) Surface-Enhanced raman spectroscopy and nano geometry: The plasmonic origin of SERS. J Phys Chem C 111(49): 17985-17988.
- Zhicheng L, Weidong R, Jingxiu Y, Weiqing X, Chun Z, et al. (2009) Deposition of Ag nanoparticles on porous anodic alumina for surface enhanced Raman scattering substrate. J Raman Spectroscopy 40(1): 112-116.
- Terekhov SN, P Mojzes, SM Kachan, NI Mukhurov, SP Zhvavyi, et al. (2011) A comparative study of surface-enhanced Raman scattering from silver-coated anodic aluminum oxide and porous silicon. J Raman Spectroscopy 42: 12-20.
- Dlugunovich VA, NI Mukhurov, Zhumar AY (2018) Evaluation of optical anisotropy of nanoporous alumina films by stokes-polarimetry and matrix coherence methods. Journal of Applied Spectroscopy 85: 936-941.
© 2020 Mukhurov N. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






