- Submissions

Full Text
Determinations in Nanomedicine & Nanotechnology
Development of DNA Nanotechnology for Cancer Therapy
Ahmed M Abu Dief1,2*
1Chemistry Department, Faculty of Science, Egypt
2Chemistry Department, Faculty of Science, Saudi Arabia
*Corresponding author: Ahmed M Abu Dief, Faculty of Science, Egypt, Saudi Arabia
Submission: December 03, 2019;Published: December 09, 2019

ISSN: 2832-4439 Volume1 Issue4
Abstract
Cancer is one of the main causes of worldwide mortality, due to the absence of precise diagnostic tools for the cancer early stages. Thus, early diagnosis, which supplies important information for therapy of cancer timely, is of great prominence for propagation of cancer cells, controlling the growth of the disease, and improving of patients survival rates. To attain the goals of early diagnosis and cancer therapy timely, nanotechnology of DNA can be effective, since it has protruded as a suitable technique for the fabrication of different nanoscale devices and structures. The eventual DNA-based nanoscale devices and structures show wonderful performance in diagnosis of cancer, owing to their small sizes, structures, high programmability and biocompatibility. In particular, the quick development of DNA nanotechnologies, like molecular association technologies, confers DNA-based nanomaterials with more intellectualization and functionalization. In DNA nanotechnology, Watson-Crick DNA molecules are arranged into variety of nanostructures in the range of 10-100nm size under special physical conditions due to electrostatic attraction between free electrons of base nitrogen and phosphate oxygen and sugar. Here, i summarize recent advances made in the development of DNA nanotechnology for the fabrication of intelligent and functional nanomaterials and highlight the potentials of this technology in diagnosis and therapy of cancer
Keywords:Nanomedicine; Nanotechnology; Cancer; Tumor cells; Drug delivery; DNA; Origami
Introduction
Cancer, a major and increasing worldwide health problem, has already become the second
leading cause of death in recent years. As a prime, yet unmet threaten to healthcare globally,
cancer gives rise to around 9 million deaths each year in the whole world [1,2]. In order to
increase the rate survival of cancer patients, timely therapy and early diagnosis become highly
substantial to improve the prognosis of cancer patients, particularly patients who have breast
cancer. Drug delivery of molecules specifically to the tumor site is an exigent demand to avoid
side effects during therapy of cancer.
The foundation for nano-medicine paths were pioneered by the earlier discoveries:
A. Controlled release system of macromolecules.
B. Liposomes in drug delivery and in transfection of DNA [3].
C. Revolving stealth polymeric nanoparticles [4].
D. Quantum dot bio-conjugate system [5] and
E. Nanowire nano-sensor dates [6].
In addition, for therapy of cancer, researchers have been improving anti-cancer drug
delivery systems to target tissues or tumor cells more precisely and produce less side effects
compared with chemotherapy [7]. In order to conquer the challenges aforementioned,
DNA has drawn a lot of interest, owing to its predictable small size, secondary structure,
high programmability, and biocompatibility [8]. Moreover, DNA nanotechnology, a
technique applying the biomolecular self-assembly property of DNA, has a broad range of
implementations in different disciplines, especially in drug delivery, synthetic biology, and
chemical analysis [9].
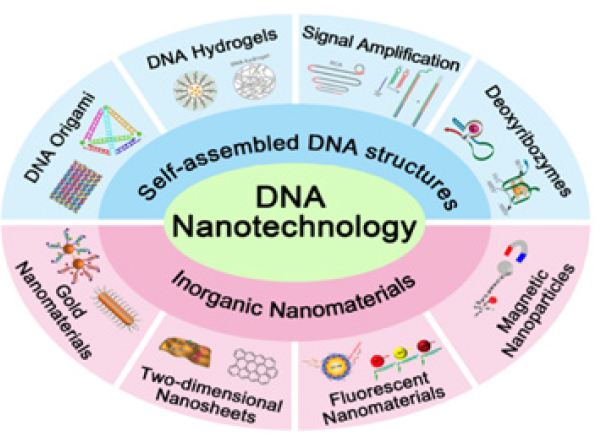
As a portentous diagnostic and therapeutic nanoplatform, DNA strands combined with other nano materials, such as nanosheets, nanotubes, nanowires, gold nanoparticles (AuNPs), polymers, iron oxides, and quantum dots show a great potential in timely therapy and early diagnosis of cancer [10,11]. This review summarizes modern progress in the development of DNA nanotechnology as shown in Figure 1 and deals with the application of nanotechnology of DNA in intelligent nanomaterials and synthesizing functional for diagnosis and therapy of cancer.
Figure 1: Schematic illustration of different applications associated with DNA nanotechnology.
Preceding Nanostructures of DNA
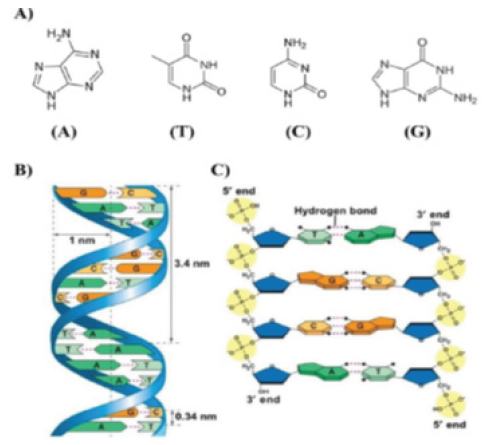
The chemical and physical properties of DNA nanostructures
are registered in excellent published reviews [12-14]. Here, we
describe the main elements that are beneficial to understand
the self-assembled nanostructure of DNA as nanocarriers drug.
The purine and pyrimidine bases that form the nucleotides in
single-stranded DNA are attached to pentose sugar and this latter
associated unit is known by a nucleoside, which is connected to
another nucleoside by the phosphodiester bond. The asymmetric
ends of DNA strands are called the ‘3 and 5’ ends depending on
whether the terminal group is a free hydroxyl group or a phosphate
group respectively. The purine bases are categorized into two
types: Guanine (G) and Adenine (A). They have a structure derived
from the fusion of six- and five- membered heterocyclic structure,
while the pyrimidines are Cytosine (C) and Thymine (T) are sixmembered
ring (Figure 2A).
In B-form dsDNA, the most prevalent form of double helix,
two nucleotide nanowires are twisted around each other with a
replicate unit every 3.4nm while maintaining a distance of 3.4Å
between the successive base pairs in a double helix with a diameter
of 2nm (Figure 2B,2C).
Figure 2:A. Adenine (A), Thymine (T), Cytosine (C) and Guanine (G) of DNA responsible for the robust
complementary base pair interactions between DNA strands.
B. Key features of DNA structures.
C. Chemical structure of DNA stabilized by hydrogen bonds between the bases A-T and G-C.
DNA Origami-Based Theranostic Nanoplatform
Nanotechnology of DNA uses the molecular confession properties of DNA to create artificial structures of DNA for technological purposes. DNA origami, a self-assembled structure, is capable of localizing hybridization reactions of DNA on three-dimensional (3D) self-assembled nanostructures or twodimensional (2D) lattices. Origami nanostructure of DNA is regarded considered as a nanoplatform that can give chances to develop a large number of applications, including drug delivery for cancer therapy and biosensing for cancer diagnosis [15-17]. DNA tetrahedron, a 3D self-assembled DNA origami nanostructure, is widely used as a sensitive biosensing probe which can be rapidly internalized by a caveolin-dependent pathway. Moreover, DNA origami-based molecular recognition elements can be incorporated with anti-cancer drugs to display exerts therapeutic effects on cancer and specific location information of cancer cells at the same time. Doxorubicin hydrochloride (Dox) is well recognized as an operative anti-cancer drug due to its ability of intercalating into C-G base pairs and strong affinity toward double helix of DNA [18,19], so it is widely used as cancer agent.
DNA-Based Drug Delivery
Several DNA-based nanostructures, namely icosahedral,
tetrahedral [20], nanotube [21,22], triangle and square have
been developed recently for in vitro and in vivo drug delivery
applications. In contrast to dsDNA, nanostructures of DNA could be
internalized within the cells without any support from transfection
agents [23] and, when densely tinned could be effectively used for
the drug delivery objectives [24]. The cellular localization of DNA
origami could be discovered by fluorescence-based assays, which
have the disadvantage of employing fluorescent labels. As alternate
path, Okholm et al. [25] use Quantitative real time Polymerase
Chain Reaction (QPCR) of M13 amplicons to quantify the cellular
absorption of origami structures of DNA [25]. In order to improve
and enhance the cell transfection/cellular uptake of DNA-based
systems, Mikkilä et al. [26] elucidated the possibility of coating
DNA origami via virus capsid proteins, which can self-assemble and
bind on the surface of origami through electrostatic interactions
and bundle the DNA nanostructures inside the capsid [26]. More
recently Brglez et al. [27] designed an intercalator based on acridine
derivatives that increased the cell uptake compared to unmodified
origami and adjust the surface properties of DNA nanostructures
[27].
Tetrahedral structures of DNA have also been illustrated to
conserve single-strand sequences against nuclease degradation;
especially, this kind of structure has been utilized to increase the
in vivo circulation half-time of siRNA from 6 to 24 minutes (N3)
[28] and deliver Guanosine-phosphate-(Cytosine GpC) to reduce an
immune response (N4) [29]. Finally, highly biocompatible tetheredaptamer
nano train of DNA (N5) versus folic acid receptor showed
high antitumor efficacy and minimize the side effects of doxo in a
mouse xenograft tumor model [30]. Recent studies show that a halficosahedral
nanostructure (N6) can effectively delivery doxorubicin
to hepatic and breast cancer cells. The study displayed that the
importance of structure and the shape of DNA nanostructures for
biomedical applications [31].
Conclusion
The applications of DNA nanotechnology were reviewed in this paper. Through Watson-Crick base pair of DNA can selfassemble to become a functional nanostructure, which has been vastly used for targeted drug delivery and imaging of cancer cells. Moreover, by appointing a various of nanomaterials, such as, fluorescent nanoparticles, nanosheets, gold nanomaterials and magnetic nanoparticles, DNA can be more efficiently utilized in cancer therapy and diagnosis, with minimize risk to be degraded by intracellular nuclease. In the near future, it is assured that more and more intelligent and functional nanomaterials based on DNA will be developed for cancer therapy and diagnosis. Despite the ingrained disadvantages of DNA-based nanomaterials, such as poor efficacy of transport, induction of undesired immune responses, and poor stability in the tumor environment, great advances have already been made to cope these challenges so to achieve early tumor diagnosis and accurate treatment in a near future. In the meantime, it is clear that more efforts should also be dedicated for the improvement of the stability and safety of DNA-based nanomaterials.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. (2011) Global cancer statistics. CA Cancer J Clin 61(2): 69-90.
- Siegel RL, Miller KD, Jemal A (2019) Cancer statistics. CA Cancer J Clin 69(1): 7-34.
- Langer R, Folkman J (1976) Polymers for the sustained release of proteins and other macromolecules. Nature 263(5580): 797-800.
- Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, et al. (1994) Biodegradable long-circulating polymeric nanospheres. Science 263(5153): 1600-1603.
- Chan WC, Nie S (1998) Quantum dot bioconjugates for ultrasensitive non isotopic detection. Science 281(5385): 2016-2018.
- Cui G, Yoo JH, Woo BW, Kim SS, Cha GS, et al. (2001) Disposable amperometric glucose sensor electrode with enzyme-immobilized nitrocellulose strip. Talanta 54(6): 1105-1111.
- Hartshorn CM, Bradbury MS, Lanza GM, Nel AE, Rao J, et al. (2018) Nanotechnology strategies to advance outcomes in clinical cancer care. ACS Nano 12(1): 24-43.
- Zhu X, Chen X, Ban F, Cao Y, Zhao J, et al. (2017) The design of a mechanical wave-like DNA nanomachine for the fabrication of a programmable and multifunctional molecular device. Chem Commun 53(76): 10504-10507.
- Brodin JD, Sprangers AJ, McMillan JR, Mirkin CA (2015) DNA-mediated cellular delivery of functional enzymes. J Am Chem Soc 137(47): 14838-14841.
- Llevot A, Astruc D (2012) Applications of vectorized gold nanoparticles to the diagnosis and therapy of cancer. Chem Soc Rev 41(1): 242-257.
- Wang P, Rahman MA, Zhao Z, Weiss K, Zhang C, et al. (2018) Visualization of the cellular uptake and trafficking of DNA origami nanostructures in cancer cells. J Am Chem Soc 140(7): 2478-2484.
- Jones MR, Seeman NC, Mirkin CA (2015) Nanomaterials. Programmable materials and the nature of the DNA bond. Science 347(6224): 1260901-1260911.
- Saccà B, Niemeyer CM (2012) DNA origami: The art of folding DNA. Angew Chem Int Ed Engl 51(1): 58-66.
- Pinheiro AV, Han D, Shih WM, Yan H (2011) Challenges and opportunities for structural DNA nanotechnology. Nat Nanotechnol 6(12): 763-772.
- Powell JT, Akhuetie OBO, Zhang Z, Lin C (2016) DNA origami rotaxanes: Tailored synthesis and controlled structure switching. Angew Chem Int Ed 55(38): 11412-11416.
- Hong F, Zhang F, Liu Y, Yan H (2017) DNA origami: Scaffolds for creating higher order structures. Chem Rev 117(2): 12584-12640.
- Zheng H, Xiao M, Yan Q, Ma Y, Xiao SJ (2014) Small circular DNA molecules act as rigid motifs to build DNA nanotubes. J Am Chem Soc 136(29): 10194-10197.
- Leach JC, Wang A, Ye K, Jin S (2016) A RNA-DNA hybrid aptamer for nanoparticle-based prostate tumor targeted drug delivery. Int J Mol Sci 17(3): 380.
- Cho Y, Lee JB, Hong J (2014) Controlled release of an anti-cancer drug from DNA structured nano-films. Sci Rep 4: 4078.
- Zhang C, Su M, He Y (2008) Conformational flexibility facilitates self-assembly of complex DNA nanostructures. Proc Natl Acad Sci 105(31): 10665-10669.
- Yan H, Park SH, Finkelstein G (2003) DNA-templated self-assembly of protein arrays and highly conductive nanowires. Science 301(5641): 1882-1884.
- Mathieu F, Liao S, Kopatsch J (2005) Six-helix bundles designed from DNA. Nano Lett 5(4): 661-665.
- Walsh AS, Yin H, Erben CM, Wood MJ, Turberfield AJ (2011) DNA cage delivery to mammalian cells. ACS Nano 5(7): 5427-5432.
- Li J, Pei H, Zhu B, Liang L, Wei M, et al. (2011) Self-assembled multivalent DNA nanostructures for noninvasive intracellular delivery of immunostimulatory CpG oligonucleotides. ACS Nano 5(11): 8783-8789.
- Okholm AH, Nielsen JS, Vinther M, Sørensen RS, Schaffert D, et al. (2014) Quantification of cellular uptake of DNA nanostructures by qPCR. Methods 67(2): 193-197.
- Mikkilä J, Eskelinen AP, Niemelä EH, Veikko L, Mikko JF, et al. (2014) Virus-encapsulated DNA origami nanostructures for cellular delivery. Nano Lett 14(4): 2196-2200.
- Brglez J, Nikolov P, Angelin A, Niemeyer CM (2015) Designed intercalators for modification of DNA origami surface properties. Chem Eur J 21(26): 9440-9446.
- Lee H, Lytton JAKR, Chen Y, Love KT, Park AI, et al. (2012) Molecularly self-assembled nucleic acid nanoparticles for targeted in vivo siRNA delivery. Nat Nanotechnol 7(6): 389-393.
- Liu X, Xu Y, Yu T, Craig C, Yan L, et al. (2012) A DNA nanostructure platform for directed assembly of synthetic vaccines. Nano Lett 12(8): 4254-4259.
- Zhu G, Zheng J, Song E, Donovan M, Zhang K, et al. (2013) Self-assembled, aptamer-tethered DNA nano trains for targeted transport of molecular drugs in cancer theranostics. Proc Natl Acad Sci U S A 110(20): 7998-8003.
- Kumar V, Bayda S, Hadla M, Caligiuri I, Russo SC, et al. (2016) Enhanced chemotherapeutic behavior of open-caged DNA @doxorubicin nanostructures for cancer cells. J Cell Physiol 231(1): 106-110.
© 2019 Ahmed M Abu Dief. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






