- Submissions

Full Text
Determinations in Nanomedicine & Nanotechnology
A Theoretical Study on the Structure and Spectral Characteristics of Various TiO2 Nanoparticles
Smirnova OV*
Chuiko Institute of Surface Chemistry, Ukraine
*Corresponding author: Chuiko Institute of Surface Chemistry, Ukraine
Submission: October 1, 2019;Published: November 21, 2019

ISSN: 2832-4439 Volume1 Issue3
Introduction
One of the promising materials for photocatalysis is titanium dioxide TiO2, the interest to it is due to its physicochemical properties. In addition, it is used as an effective photocatalyst for a number of chemical reactions and is widely appied to purifying water and air from toxic organic impurities. Interest in the theoretical study of the interaction of O2 molecules, which in the gas phase are in the form of a triplet diradical, with a solid-phase surface, has grown significantly. The results of such studies are fundamental both for adsorption and catalysis, and for the general theory of metal corrosion, and let it possible to eliminate the problem considerably, at least to slow down the corresponding reactions [1].
Molecular oxygen absorbs radiation very weakly in the spectral range from infrared to ultraviolet, howeVer, the influence of the molecular environment makes it possible for O2 molecules to exist in the singlet state (α1Δg). the formation of which as photoexcited is prohibited in the gas phase. O2 molecules (α1Δg), due to their high reactivity, play a key role in natural photobiological and photochemical processes, what actualizes a detailed study on the mechanism of formation of singlet oxygen.
In the cross section of the potential energy surface was calculated along the direct approximation of the O2 molecule with a Ti14H22O40 cluster, which imitates the most characteristic part of the anatase surface separately for the singlet and triplet states [2]. It has been found that adsorption of molecular oxygen on the defect-free (001) face of anatase does not occur, and an adsorption on the oxygen vacancy mentioned above is preceded by a triplet-singlet transition in the “001+O2 cluster” system [3].
The calculations were carried out in the framework of the cluster approximation. using the density functional theory B3LYP method and the basis set 6-31G (d,p) using the PC GAMESS software package (FireFly version 8.1.0, http://classic.chem.msu.su/gran/firefly/index.html by A Granovsky). Also, to simulate the effect of atomic nitrogen incorporation and isomorphic replacement of a Ti atom by a Zr one, anatase nanoparticles were represented as a set of clusters [4]. The (001) face of a defect-free anatase surface was modeled by a cluster of the gross formula Ti14H22O39 (cluster A). The presence of an oxygen vacancy was reproduced by the Ti14H22O38 cluster obtained by removing one double-coordinated surface oxygen atom from th cluster A (Figure 1).
TiO2 clusters doped with both N and Zr atoms (Ti13ZrH22O39, Ti13ZrN2H22O37, Ti13ZrN2H22O36) were also considered (Figure 2). All the oxygen atoms in a defect-free structure (Figures 1 & 2) can be divided into some groups according to their coordination environment (2-and 3-coordinated ones) and the chemical nature of the second and third neighboring atoms. Five peaks of O1s can be observed in the XPS spectrum calculated for the Ti14H22O39 cluster model (about 521eV). Each peak can be assigned to a specific type of O atoms, shown in the inset to Figure 3.
Figure 1:Molecular models of defect less titanium dioxide Ti14H22O39 (a) and a defect of substitution of titanium with nitrogen: Ti14N2H22O37 (b) and Ti14N4H22O35 (c) in a titanium dioxide cluster.
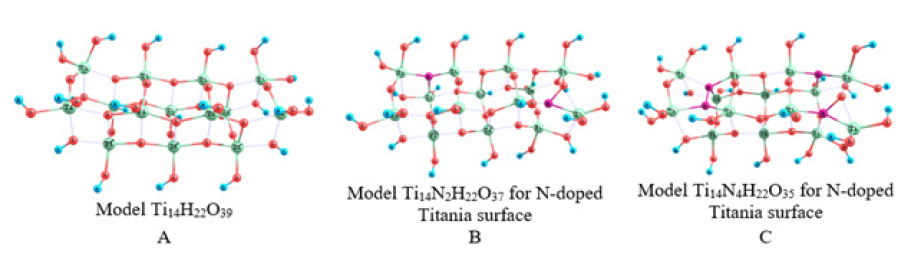
Figure 2:Molecular models of the substitutional defect of titanium with zirconium Ti13ZrH22O39 in a titanium dioxide cluster.
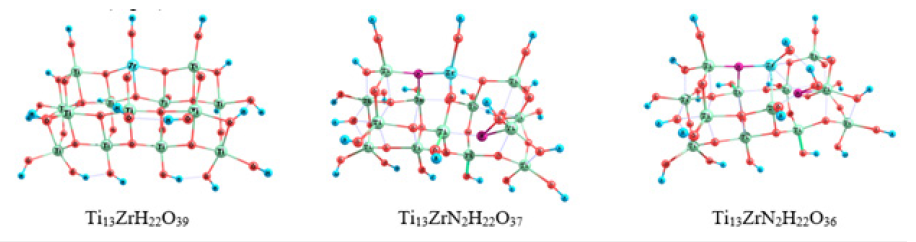
Figure 3:Theoretically calculated XPS spectrum of the Ti14H22O39 cluster. Schemes of various types of chemical environment of O atoms and respective positions of the O1s peak.
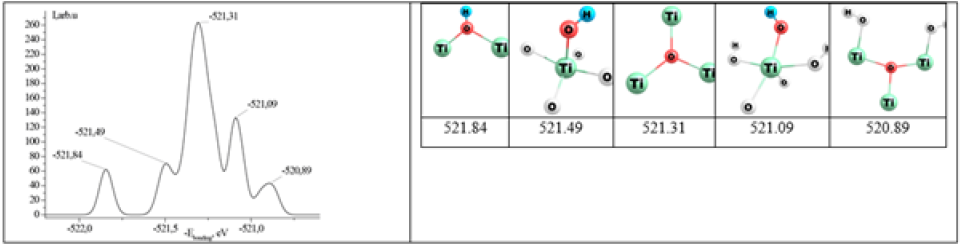
Removal of the oxygen atom from the matrix of titanium dioxide leads to the splitting of peaks from 2-and 3-coordinated oxygen atoms (521.44 and 521.28eV, respectively). Replacing oxygen atoms with nitrogen atoms complicates the spectrum (at least 12 peaks are observed). However, 2 new peaks appear near 523eV (522.87 and 523.28eV) associated with 3-coordinated oxygen atoms. Simultaneous nitrogen substitution of oxygen atoms and an oxygen vacancy complicate the XPS spectrum even more: 15 peaks can be distinguished, including a pair of about 523eV, namely 522.60 and 522.74eV. Various structures of adsorption complexes of water molecules are also considered, one of which is associated with the formation of the HOH...O hydrogen bond, while the other is characterized by the coordination bond Ti...OH2. In the first case, the shift of the O1s line is approximately 0.05eV and is the same for all peaks of the spectrum calculated for the adsorption complex.
In addition, cluster models of titanium dioxide were studied, including more than 21 Ti atoms (pure and doped with nitrogen). It has been shown that an increase in the number of atoms in the model does not contribute to improving the agreement between experimental and theoretical data [5].
References
- Heller A, Jarvis K, Coffman SS (2018) Association of type 2 diabetes with submicron titanium dioxide crystals in the pancreas. Chem Res Toxicol 31(6): 506-509.
- Smirnova O, Grebenyuk AG, Lobanov VV (2016) Quantum chemical calculations on adsorption of O2 molecules on the anatase (001) surface. Surface 8(23): 73-77.
- Smirnova O, Grebenyuk A, Lobanov V (2017) Titanium dioxide defect structures as catalytic sites. Surface 9(24): 44-56.
- Smirnova O, Grebenyuk A, Lobanov V (2017) Theoretical investigation of pollutant species adsorption on oxygen vacancies of pure and nitrogen-doped titania. Bioactive Materials 24-25:
- Mo S, Ching W (1995) Electronic and optical properties of three phases of titanium dioxide: Rutile, anatase, and brookite. Phys Rev B 51(19): 13023-13032.
© 2019 Smirnova OV. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






