- Submissions

Full Text
Determinations in Nanomedicine & Nanotechnology
Optimizing the Annealing Effect of Zn/Ac Nanoparticle Synthesis on Dye Wastewater Treatment by Combination of Ultrasonic and Photocatalytic Methods
Behnoosh Khataei1* and Mahdi ghaderi2
1Department of Civil and Environmental Engineering, Iran
2Department of Mechanical Engineering, Iran
*Corresponding author: Behnoosh Khataei, Department of Civil and Environmental Engineering, Iran
Submission: October 09, 2019;Published: October 15, 2019

ISSN: 2832-4439 Volume1 Issue3
Opinion
Many different environmental researches were done on the treatment of water, wastewater and air pollutants by different biological, physical and chemical processes. Pollution of water sources by dye pollutants from various industries such as textile, paper, rubber and plastic industries is a major environmental problem. Organic dyes cause irreparable damage to the environment due to the avoiding the light entry to water, disruption of photosynthesis, reduction of oxygen transfer to water, occurrence of eutrophication, interference with the ecology of the receiving waters, toxic effects as well as unpleasant appearance [1-18]. Different methods are used for the treatment of dye wastewater [19-25].
One of the new methods in dye wastewater treatment is oxidation using cavitation and ultrasound as well as sono-electrochemical methods. In fact, the photocatalytic oxidation process by visible or UV light in the presence of catalysts such as titanium dioxide or zinc oxides one of the advanced oxidation processes in the removal of organic pollutants, that is more efficient than other processes [26]. Also, using the ultrasonic and acoustic wave motion in the aquatic environment oscillates the molecules, which creates contractile and expansion cycles [27,28], breaking the bonds and thus accelerating the purification process.
In this study, the combination of photocatalytic and ultrasonic methods in the treatment of dye wastewater was investigated. The studied catalyst is zinc oxide which is highly resistant to light and chemical corrosion. Also, it was applied for oxidizing the wide range of organic compounds due to its non-toxicity, insolubility, ability to decompose toxic organic compounds, ability to absorb a wide range of electromagnetic waves and the photocatalytic capability for radiation.
We used the response surface methodology, the best multivariate techniques [29]. For this purpose, Visible Light Source (W), Ultrasonic Power (W), Dye Concentration (mg/L), pH, Synthesis Temperature (C), nano-particle concentration (gr/L) as variable and percentage of dye removal as the answer were considered.
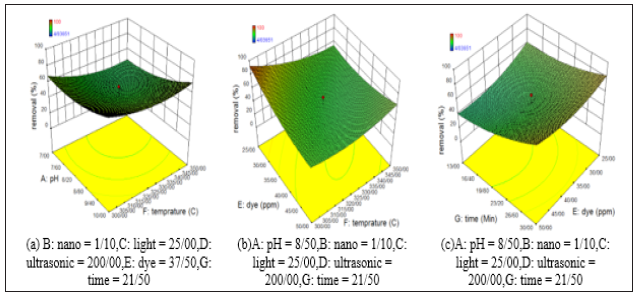
In this study, a cubic reactor with dimensions of 30 * 30 * 20cm Figure 1, incandescent lamps and ultrasonic device with the power 0 to 400w and 20KHz driving frequency were used. Zn(CH3COO) 2.2H2O and Poly Vinyl Pyrrolidone (PVP) manufactured by Merck were used for this synthesis. The catalyst synthesis was performed at various temperatures (300, 325, 284.33, 350 and 365.66 °C). The synthesized nanoparticles had almost similar colors and were close to dark gray. Finally, the optimum temperature was determined after photocatalytic tests were performed on each of these nanoparticles in the presence of ultrasound and visible light. In this study, the model was presented based on response surface methodology tests using Design Expert 10 software and Quadratic model to evaluate the nonlinear behavior of the results. The target contaminant is methylene blue dye with the chemical formula of C16H18N3SCl and molecular weight of 319.85gr/mol made by Merck, Germany. It is a cationic dye that has a pH=3 at 20 °C and is one of the aromatic chemical dyes. For this reason, it is carcinogenic, mutagenic, often toxic and resistant to biodegradation. According to the results, the simultaneous effect of the variables on the dye removal percentage was investigated presented in Figure 2.
As can be seen, the higher synthesis temperature caused the lower removal rate; The better synthesis temperature for the ZnO nanoparticles is 300 °C. The above nanoparticles also perform better at the alkaline pH (about 10) and the removal rate increases Figure 2(a). According to Figure 2(b), as the dye concentration decreases, the removal percentage also increases. By increasing the synthesis temperature, the removing ability of the nanoparticles and consequently the dye removal percentage decreased. So, the optimum synthesis temperature and contaminant concentration is 300 °C and 25ppm. On the other hand, it observed a better removal percentage in 30 minutes because the nano-particle bonds broke under irradiation and ultrasound waves and produced more negative hydroxyl. So, higher removal rates could be observed at higher times. As shown in Figure 2(c), the optimum time and dye concentrations 30 minutes 25ppm. Also, the interaction graphs showed the antagonistic effect of pH on temperature and temperature on dye concentration. But time has a synergistic effect on the color concentration pollutant removal percentage.
Figure 1:The schematic of used reactor.
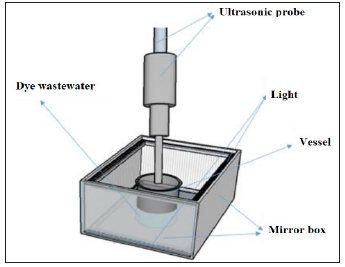
Figure 2:Simultaneous effect of variables on removal percentage.
References
- Wang J, Li C, Zhuang H, Zhang J (2013) Photocatalytic degradation of methylene blue and inactivation of Gram-negative bacteria by TiO2 nanoparticles in aqueous suspension. Food Control 34(2): 372-377.
- Qaderi F, Ayati B, Ganjidoust H (2011) Role of moving bed biofilm reactor and sequencing batch reactor in biological degradation of formaldehyde wastewater. Journal of Environmental Health Science and Engineering 8(4): 295-306.
- Qaderi F, Babanezhad E (2017) Prediction of the groundwater remediation costs for drinking use based on quality of water resource, using artificial neural network. J Clean Prod 161: 840-849.
- Qaderi F, Sayahzadeh AH, Azizi M (2018) Efficiency optimization of petroleum wastewater treatment by using of serial moving bed biofilm reactors. J Clean Prod 192: 665-677.
- Babanezhad E, Amini RH, Hosseini KSS, Qaderi F (2017) Investigating nitrogen removal using simultaneous nitrification-Denitrification in transferring wastewater through collection networks with small-diameter pipes. Water Pract Technol 12(2): 396-405.
- Babanezhad E, Qaderi F, Salehi ZM (2018) Spatial modeling of groundwater quality based on using Schoeller diagram in GIS base: A case study of Khorramabad, Iran. Environ Earth Sci 77: 339.
- Qaderi F, Sayahzadeh AH, Azizpour F, Vosughi P (2019) Effciency modeling of serial stabilization ponds in treatment of phenolic wastewater by response surface methodology. International Journal of Environmental Science and Technology 16(8): 4193-4202.
- Ebrahimi GM, Qaderi F, Babanezhad E (2018) Prediction of mortality resulted from NO2 concentration in Tehran by Air Q+ software and artifcial neural network. International Journal of Environmental Science and Technology 16(3): 1351-1368.
- Sheikholeslami Z, Yousefi KD, Qaderi F (2018) Nanoparticle for degradation of BTEX in produced water; an experimental procedure. Journal of Molecular Liquids 264: 476-482.
- Sheikholeslami Z, Yousefi KD, Qaderi F (2018) Investigation of photocatalytic degradation of BTEX in produced water using γ-Fe2O3 Journal of Thermal Analysis and Calorimetry 135(3): 1617-1627.
- Faghih NE, Yousefi KD, Qaderi F (2018) An experimental study on the simultaneous phenol and chromium removal from water using titanium dioxide photocatalyst. Civil Engineering Journal 4 (3): 585-593.
- Yavari SM, Qaderi F (2018) Determination of thermal pollution of water resources caused by Neka power plant through processing satellite imagery. Environment Development and Sustainability pp. 1-23.
- Tamadoni A, Qaderi F (2019) Optimization of soil remediation by ozonation for PAHs contaminated soils. Ozone: Science & Engineering 41(5): 454-472.
- Tavakoli MM, Qaderi F (2019) Modeling of petroleum wastewater treatment by Fe/Zn nanoparticles using the response surface methodology and enhancing the efficiency by scavenger. Results in Physics 15: 102566-102576.
- Asadi P, Amini RH, Qaderi F (2019) Comparison of Chlorella vulgaris and Chlorella sorokiniana91 in post treatment of dairy wastewater treatment plant effluents. Environmental Science and Pollution Research, USA.
- Khalegh R, Qaderi F (2019) Optimization of the effect of nanoparticle morphologies on the cost of dye wastewater treatment via ultrasonic/photocatalytic hybrid process. Applied Nanoscience pp. 1-21.
- Qaderi F, Asadi P, Tamadoni A, Azizi M (2018) Evaluation of sustainability of development in zone 22 of Tehran by ecological footprint method. Geography and Development Iranian Journal 16(50): 231-245.
- AzizpourF, Qaderi F (2019) Optimization modeling and uncertainty investigation of phenolic wastewater treatment by photocatalytic process in cascade reactor. Environment Development and Sustainability, Netherlands.
- Dejohn PB, Hutchins RA (1976) Tex Chem Color 8: 69.
- Patil SS, Shinde VM, (1988) Biodegradation studies of aniline and nitrobenzene in aniline plant wastewater by gas chromatography. Environ Sci Technol 22(10): 1160-1165.
- More AT, Vira A, Fogel S (1989) Biodegradation of trans-1,2-dichloroethylene by methane-utilizing bacteria in an aquifer simulator. Environ Sci Technol 23(4): 403-406.
- Slokar YM, Marechal LAM (1998) Methods of decoloration of textile wastewaters. Dyes Pigments 37(4): 335-336.
- Ortiz I, Mosquera CA, Rodicio JL, Esplugas S (2015) Advanced technologies for water treatment and reuse. AIChE J 61(10): 3146-3158.
- Jadhav SV, Bringas E, Yadav GD, Rathod VK, Ortiz I, et al. (2015) Arsenic and fluoride contaminated groundwaters: A review of current technologies for contaminants removal. J Environ Manage 162: 306-325.
- Fernández CP, Vallejo M, Román SMF, Ortiz I (2015) Insight on the fundamentals of advanced oxidation processes. Role and review of the determination methods of reactive oxygen species. J Chem Technol Biotechnol 90(5): 796-820.
- Kamat PS, Huehn R, Roxana N (2008) Semiconductor nanostructures for simultaneous detection and degradation of organic contaminants in water. Photochem Photobiol Chem 42: 37-57.
- Ashok KM, Grieser F (1991) Ultrasonic assisted chemical processes. Rev Chem Eng 1: 123-129.
- Stack JL, Carney AP, Malone BH, Wessels KT (2003) Factors influencing the ultrasonic separation of oil-in-water emulsions. Ultrasonics Sonochemistry 12(3): 153-160.
- Lundstedt T, Seifert E, Abramo L, Thelin B, Nystrom A, et al. (1998) Experimental design and optimization. Chemometr Intell Lab Syst 42: 3-40.
© 2019 NA Shah. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






