- Submissions

Full Text
Degenerative Intellectual & Developmental Disabilities
Strategies to Reduce Cerebral Palsy in Perinatal Medicine
Kazuo Maeda*
Department of Obstetrics and Gynecology, Tottori University Medical School, Japan
*Corresponding author: Kazuo Maeda, Department of Obstetrics and Gynecology, Emeritus, Tottori University Medical School, Tottoriken, Japan
Submission: October 10, 2017;Published: April 20, 2018

Volume1 Issue3April 2018
Abstract
Aims: To prevent fetal brain damage and cerebral palsy (CP).
Methods: FHR score was studied with objective FHR changes. The FHR and fetal movements were recorded in actocardiogram (ACG). Computerized diagnosis, hypoxia index and GLHW, clinical ultrasound tissue characterization, were used for the diagnosis.
Result: FHR increased when fetus moved, and hypoxic damage was shown by the loss of acceleration followed by the loss of variability. Physiologic sinusoidal was diagnosed by actocardiogram. Caesarean delivery at the loss of acceleration and reduced variability will reduce cerebral palsy. Sum of duration of repeated FHR decelerations was main component of hypoxia index (HI). The cerebral palsy (CP) will be prevented by the early delivery performed if the HI is less than 25. Fetal growth restriction and asphyxia were treated by maternal heparin therapy in the case of placental intervillous space fibrin deposit, detected by high placental GLHW. Fetal brain periventricular echodensity (PVE), diagnosed by GLHW, followed by neonatal PVL and CP, disappears in the full term delivery, or possibly treated in neonatal stage. Late deceleration, the deceleration in supine hypotension, and umbilical cord abnormality will disappear by maternal lateral posture.
Conclusion: There are cases cured by Caesarean delivery, while some abnormalities will be treated without surgery.
Keywords: FHR; CTG; Actocardiogram; Acceleration; Variability; FHR score; Hypoxia index; Brain damage; Cerebral palsy
Introduction
Objective study was opened in obstetrics since 1950s. The first medical electronics was the author handmade electroencephalography [1], abdominal lead fetal electrocardiography (FECG), phonocardiography (FPCG) and neonatal respirography [2].
Figure 1: Although the first record recorded fetal P and T in simple abdominal lead fetal ECG, fetal P and T were masked by noises in later simple abdominal lead FECG.
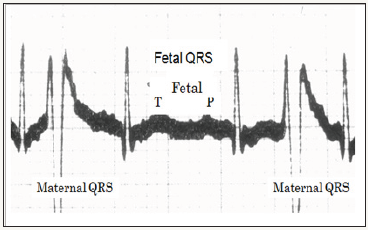
Abdominal Lead Fetal Electrocardiogram (FECG)
The first abdominal lead fetal ECG recorded fetal P and T (Figure 1) suggesting possibility of fetal electrocardiography. However, fetal P and T waves were masked by muscular action potentials; thus, simple abdominal lead FECG was unable to diagnose fetus [3]. Although low noise fetal ECG was obtained by fetal scalp lead, its needle electrode was risky to promote infection of maternal viral diseases [4].
Fetal Phonocardiography (FPCG)
Although fetal condition was estimated listening to fetal heart tone using stethoscope in old time, it was subjective diagnosis. Later, fetal heart tone was listened the most clearly by high-pitched fetal heart tone, it failed to detect sinusoidal FHR before IUFD in 1960s, thus, we discarded listening to fetal heart beats, and moved to fetal heart rate (FHR) recorded.
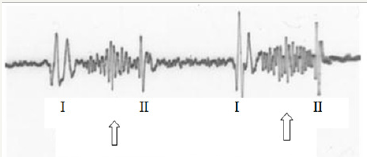
Fetal heart tone was recorded on chart (FPCG) above 80 Hz to by the cardiotocogram (CTG) triggered with fetal heart tones to detect abnormal fetal heart,however, the record was disturbed by false systolic murmurs recorded in 16% of normal fetus (Figure 2), which might be the sound of blood flow through ductus arteriosus, and we were unable to detect fetal pathologic heart murmur as well as congenital heart disease.
Figure 2: Fetal PCG in a normal pregnancy, where recorded systolic murmurs (arrow). We used standard PCG microphone, 80 Hz cut-off high-pass filter and displayed it on a CRT, then it was recorded by a film camera.
Methods
Cardiotocogram
We studied fetal inrapartum states with FHR and uterine contraction curves recorded on slow moving chart (cardiotocogram, CTG), visually detecting FHR changes. The first CTG, completed 1964, recorded FHR with fetal heart tone using sensitive fetal microphone triggering fetal heart rate meter in external CTG, though it was noisier than the fetal scalp lead ECG, while the needle electrode of scalp lead was risky to viral infection from the mother [5].
The external fetal monitoring changed to ultrasound Doppler autocorrelation FHR meter 1974, of which CTG was as clear as the FHR recorded by scalp lead FECG. Thus, the fetal heart tone was changed to ultrasonic autocorrelation fetal heart rate meter, which is main use in external CTG at present. However, the FHR deceleration classification with visual recognition into periodic and variable changes followed by early, late and variable decelerations [6] were subjective resulting vague decision and inter-observer difference, and also the pattern classification was difficult to explain developmental mechanism of FHR changes, e.g. no physiologic sinusoidal pattern was separated from pathologic one, the origin of heart rate irregularity (variability) was vague, variable decelerations were explained by nervous reflex in mild deceleration and hypoxia in severe variable one, i.e. they were explained by two different principles but not by single reason, which would be various grade fetal hypoxia.
Quantitative FHR evaluation using cardiotocogram
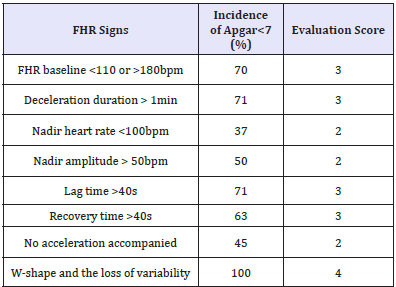
The author discarded visual pattern classification, and quantitatively analyzed FHR deceleration, baseline FHR and its variability in objectively evaluated FHR changes with the FHR score [4] (Figure 3), rejecting vague analysis and interobserver difference. The FHR score in the 1st stage of labor highly correlated to one min Apgar score and umbilical cord blood pH (Table 1), where regression equations were obtained between FHR score, Apgar score and UApH, therefore, Apgar score was expected to be 4 and UA pH was 7.07, that is acidosis, if the FHR score was 15, thus early delivery could be objectively decided, if the FHR score is high even in the 1st stage of labor.
Figure 3: Each deceleration and FHR baseline was analyzed by quantitative data.
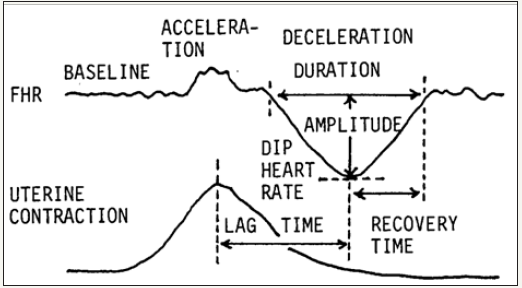
Table 1: Evaluation scores were set by the low Apgar score incidence in each FHR signs. The sum of evaluation scores in 5min is FHR score. The fetus was abnormal if FHR score was 10 or more, and severely abnormal if the score was 20 or more.
Actocardiogram (ACG)
Fetal movement was recorded in spikes with FHR and uterine contraction on the ACG [7] (Figure 4), in which various problems of CTG were solved, e.g. FHR increased when fetus moved, thus, FHR acceleration was provoked by fetal movements, physiologic sinusoidal heart rate was separated from pathologic one by its synchronization to periodically changing movements [8], and FHR variability (LTV) was provoked by minor fetal movements [9,10].
FHR changes were provoked by the reaction of fetal midbrain to fetal movement with 7sec delay due to the presence of integral function of the brain, which were proved by electronic and physiologic simulations. The acceleration was lost in early hypoxic state preserving FHR variability. The variability was lost by the most severe hypoxia forming totally flat baseline such as that of anencephaly, i.e. the loss of variability indicated the severe fetal brain damage, followed by cerebral palsy.
Figure 4: Each deceleration and FHR baseline was analyzed by quantitative data. It looks troublesome,
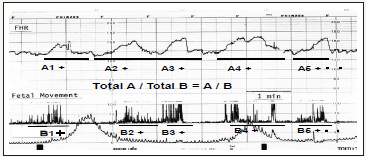
Therefore, the fetus is recommended to deliver before the loss of variability which were preceded by the loss of acceleration, variability decrease, severe bradycardia, high FHR score and high Hypoxia Index higher than 24, and so on. Neonatal brain damage followed by cerebral palsy will be prevented by the early C-delivery before the loss of variability [8,9]. Some neonatal CP will be explained by the above mechanism. Although some Japanese studies reported significant reduction of CP despite of old criteria in fetal monitoring, it would be the results achieved by the early C-delivery before unrecognized loss of variability. Physiologic sinusoidal FHR is separated from pathologic ominous sinusoidal, if the sinusoidal FHR change is synchronized with the periodic changes of fetal movements, which was mainly fetal respiratory movements [8]. Fetal hiccupping movements are continuously repeated sharp spikes with 2-3sec intervals for more than 10min, which does not accompany FHR acceleration, while there was no hypoxia, because it is an independent convulsive diaphragmatic action. It is proved by continuous sharp spikes with 2 sec intervals without formation of movement burst.
Hypoxia Index
It was controversy that fetal outcome is favorable after 2-3 late decelerations (LDs), though it is reported that LD is ominous [6], while highly repeated LDs lost variability, Apgar score was 3, and the infant was severe brain damage in the author’s experience. In addition, LD is defined after the repetition for 15min in some reports. The author estimated that the hypoxia damaged the fetus when the sum of deceleration durations is large, while hypoxia effect of 2-3 decelerations is too weak to be hazardous. Thus, the sum of duration (min) of decelerations was divided by the lowest nadir FHR (bpm), which is the intensity of hypoxia, then multiplied by 100, and the hypoxia index is obtained, which will be the same as a dip area. The heart rate is used instead of PaO2, because rabbit PaO2 closely correlate heart rate, when the PaO2 is lower than 50mm Hg [3], and human fetal PaO2 is less than 50mm Hg [4]. Therefore, Hypoxia Index is as follows;
Hypoxia index (HI) = Sum of deceleration duration (min) divided by the lowest FHR (bpm), and multiplied by 100. The HI was measured in cases of the loss of variability followed by fetal brain damage and cerebral palsy, to determine the threshold not to develop cerebral palsy (CP). HI was 25 in a case of the loss of variability followed by CP, and 26 in repeated LD for 50min, whose Apgar was 3, and the infant died in brain hemorrhage. Thus, the threshold HI not to be cerebral palsy is lower than 25. Actually, the HI was 20 to 24, smaller than 25, in cases of abnormal FHR, who associated neither the loss of variability nor cerebral palsy. Therefore, HI should be lower than 25 at delivery [9, 10]. Also, the problem of controversy LD will be solved, because the authors case of three connected LDs’ HI was 6 and the A/B ratio was 1.3 (larger than 1.0) which was normal range, and its actual Apgar score was 9.
Maternal posture
Maternal supine posture may influence fetal state. Late deceleration, the deceleration in supine hypotension, and umbilical cord compression disappeared when the mother changed to lateral posture from supine, because LD is caused by the compression of iliac artery by contracted uterus, followed by the loss of placental maternal circulation (Poseiro effect), and fetal bradycardia appears later than uterine contraction, and the LD disappears after maternal taking lateral posture .
Fetal Brain Damages in Preterm Birth Infants
Although intrapartum brain damage was such rare as one in 5,000 births, the damage was frequent in preterm birth infants [11]. Yamamoto et al reported the periventricular eho density (PVE) in preterm fetuses, which was highly echogenic periventricular B-mode zone diagnosed by high GLHW [15], and 18% of the PVE turned into neonatal brain PVL followed by CP, when the PVE lasted during pregnancy until preterm delivery. The incidence was as high as 0.2% of total births [12], Two strategies are proposed to prevent the damage;
A. Since neither PVL nor CP was found in full term birth neonates [12], the pregnancy is prolonged until term birth by any effective tocolysis, one of which will be pharmaceutical, and another will be the sedation of uterus-brain nerve in the positive feed-back loop of labor contraction by anesthetic procedure [13].
B. As the PVE lasted until preterm delivery, PVE will be found in the neonatal brain immediately after preterm birth, which is detected by ultrasound in preterm neonate, then detected PVE will be treated before the change into PVL, namely, such neonatal brain repairing material as the growth factor will be administered to the case of neonatal PVE, because the growth factor disappeared within 3-4days after birth in normal neonates [14]. The problem will be solved in the future.
Solution of Placental Fibrin Deposit in Fetal Growth Restriction and Associated Fetal Asphyxia
Fibrin deposit in placental intervillous space which hinders the intervillous maternal blood flow causing fetal growth restriction and fetal death in previous pregnancy. Placental fibrin deposit was detected by high level placental GLHW tissue characterization, where the mother was treated by 5,000U heparin infusion every day in 17 to 31st weeks of pregnancy [15].
Result
FHR score
FHR score was calculated manually in 1969, while mainly computerized calculation was common in the present.
Apgar score and UA pH was predicted using regression equations as follows;
Apgar is 7 or more, if FHR score is <10
Apgar is 6, if FHR score = 10
Apgar is 4, if FHR score = 15, UA pH will be 7.07, acidosis,
Apgar is 2, if FHR score = 20, acidosis.
Attendant doctor is alarmed by the objective and numeric diagnosis. The doctor decides early delivery by epected Apgar score and pH.
Atocardiogram
Developing mechanism of normal and pathological FHR changes were clarified by the analysis of actocardiogram, particularly, FHR acceleration, variability and their pathologic changes in hypoxia, namely, acceleration and variability are the reaction of fetal brain to fetal movements, thus, their disappeance means the weakening or the damage of fetal brain due to hypoxia, and the fetus is cured by early delivery before the loss of variability. The physiologic sinusoidal FHR is differentiated from pathologic one, the progressing fetal hypoxic damages are diagnosed, and fetal behavior is known by actocardiogram.
Hypoxia Index
Controversy late deceleration was cleared by the application of HI. Intrapartum fetal brain damage followed by infantile cerebral palsy is prevented objectively with the application of HI threshold level. The various roles of FHR decelerations in pattern classification are replaced by the single HI, therefore, the relation becomes simple, solving controversy nature of late deceleration.
Placental Fibrin Deposit
The growth restricted fetus recovered and estimated fetal weight became normal after heparin treatment. Also, fetal death was prevented and normal neonate was achieved after the heparin therapy [15]. Therefore, heparin therapy will be recommended in the fetal asphyxia of unknown cause associated with high level of placental GLHW.
Fetal Brain PVE
As the result of above described treatments to fetal brain PVE is unknown at present, therefore, it will be clarified in the future.
Discussion
Fetal heart rate diagnosis depending FHR pattern classification into early, late, mild variable, severe variable decelerations was changed to objective numeric FHR score and hypoxia index which cover the roles of various FHR deceleration patterns, including late, mild and severe variable decelerations. The addition of fetal movement to actocardiogram clarified fetal brain response to fetal movement, developing process of hypoxic FHR changes, or developing mechanism of physiologic sinusoidal FHR, and so on. The developing process of FHR changes made it simple to analyze several decelerations and analysis of hypoxic effect on the fetus.
Conclusion
Numeric analysis of FHR changes, FHR score, baseline variability, sinusoidal FHR, hypoxia index and A/B ratio will be incorporated in FHR analyzing computer in the future.
References
- Maeda K (2016) Electroencephalographic studies of eclampsia and preeclampsia. J Obstet Gynecol Res 42(1): 11-20.
- Maeda K (1954) Invention of neonatal respirograph. Obstetrics Gynecology World 6: 527-528.
- Umezawa J (1976) Studies on the relation between heart rate and PaO2 in hypoxic rabbit:comparative study for fetal heart rate change during labor. Acta Obstet Gynecl Jpn 28: 203-1212.
- Maeda K, Kimura S, Nakano H (1969) Pathophysiology of Fetus. Fukuoka Printing, Japan.
- Maeda K, Cosmi E, Gardosi J (1995) Intrapartum surveillance: recommendations on current practice and overview of new developments. FIGO Study Group on the Assessment of New Technology. International Federation of Gynecology and Obstetrics. Int J Gynaecol Obstet 49(2): 213-221.
- Hon EH (1968) An Atlas of Fetal Heart Rate Patterns. Harty Press, USA.
- Maeda K (2016) Invention of ultrasonic Doppler fetal actocardiograph and continuous recording o fetal movements. J Obstet Gynecol Res 42(1): 5-10.
- Ito T, Maeda K, Takahashi H, Nagata N, Nakajima K, et al. (1994) Differentiation between physiologic and pathologic sinusoidal FHR pattern by fetal actocardiogram. J Perinat Med 22(1): 39-43.
- Maeda K (2014) Origin of the long-term variability and acceleration of FHR studied for the prevention of cerebral palsy in fetal hypoxia and general insults. J Perinat Med 42(1): 401-403.
- Maeda K (2014) Modalities of fetal evaluation to detect fetal compromise prior to the development of significant neurological damage. J Obstet Gynecol Res 40(10): 2089-2094.
- Ishii N, Kono Y, Yonemoto N, Kusuda S, Fujimura M, et al. (2013) Outcomes of infants born at 22 and 23 weeks of gestation. Pediatrics 132(1): 62-71.
- Yamamoto N, Utsu M, Maeda K, Serizawa M, Ohki S, et al. (2000) Neonatal periventricular leukomalacia preceded by fetal periventricular echodensity. Fetal Diag Ther 15(4): 198-208.
- Maeda K (2013) A proposal to reduce congenital cerebral palsy. J Health Med Inform 4: 135.
- Tamaya T. Personal communication.
- Maeda K, Utsu M, Kihaile PE (1998) Quanification of sonographic echogenicity with grey-level histogram width: a clinical tissue characterization. Ultrasound Med Biol 24(2): 225-234.
© 2018 Kazuo Maeda. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






