- Submissions

Full Text
Developments in Clinical & Medical Pathology
Radiated and Strewn-Diffuse Large B Cell Lymphoma
Anubha Bajaj*
Consultant Histopathologist, AB Diagnostics, India
*Corresponding author:Anubha Bajaj, Consultant Histopathologist, AB Diagnostics, India
Submission: February 16, 2023;Published: February 24, 2023

ISSN:2690-9731 Volume2 Issue3
Introduction
Diffuse large B cell lymphoma not otherwise specified (NOS) is a large B cell lymphoma delineating diffuse pattern of tumour configuration. Neoplasm lacks definitive diagnostic criterion, adopted within diverse categories of large B cell lymphoma as expounded by World Health Organization (WHO). Diffuse Large B Cell Lymphoma (DLBCL-NOS) is constituted of medium to large, atypical lymphoid cells and enunciates distinctive molecular subtypes as Activated B Cell (ABC) and Germinal Centre B Cell (GCB). Diffuse large B cell lymphoma (NOS) is a non-Hodgkin lymphoma frequently arising in adults (~30%). Median age of disease occurrence is seventh decade although no age of disease emergence is exempt. A mild male predominance is enunciated. Diffuse large B cell lymphoma (NOS) commonly incriminates lymph node. Around ~40% lymphomas essentially implicate extra-nodal sites where several (~70%) instances demonstrate extra-nodal tumour occurrence ≥ singular site [1,2].
Gastrointestinal tract is a frequently incriminated extra-nodal site although no site of disease emergence is exempt. The lymphoma is commonly observed within bone marrow (16%). Diffuse large B cell lymphoma (NOS) may occur de novo or emerge therefore to transformation of low-grade lymphoma. Diffuse large B cell lymphoma (NOS) manifests as a rapidly progressive tumefaction. Majority (~75%) of neoplasms exceed ≥ 5-centimetre magnitude [1,2]. B clinical symptoms as pyrexia, drenching night sweats or loss of ≥10% body weight occur in ~one third (33%) of diffuse large B cell lymphomas (NOS). The neoplasm may infrequently be asymptomatic. Diffuse large B cell lymphoma (NOS) may represent as a transformation of pre-existing low grade B cell lymphoma as follicular lymphoma, marginal zone lymphoma, chronic lymphocytic leukaemia/small lymphocytic lymphoma, or nodular lymphocyte predominant Hodgkin’s lymphoma [3,4]. Frozen section demonstrates a neoplasm with diffuse pattern of tumour evolution. Enlarged, atypical lymphoid cells disseminated within incriminated lymph node or extra-nodal site appear consistent with large cell lymphoma. However, ancillary immuno-phenotyping and cytogenetic evaluation is mandated to establish B cell lineage and segregate specific subtypes of diffuse large B cell lymphoma [1,2]. Cytological evaluation enunciates enlarged, atypical lymphoid cells imbued with vesicular chromatin and prominent nucleoli, indicative of large cell lymphoma [3,4]. Appropriate classification of the neoplasm as diffuse large B cell lymphoma (NOS) necessitates precise immuno-phenotypic and cytogenetic assessment.
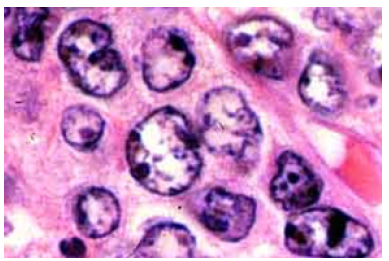
Diffuse large B cell lymphoma (NOS) exhibits partial or complete effacement of lymph node architecture along with diffuse infiltration of scattered, enlarged, or intermediate, atypical B lymphoid cells. Neoplastic cells are incorporated with vesicular chromatin and prominent nucleoli. Enlarged cells with enlarged nuclei demonstrate nuclear magnitude ≥ histiocytic nuclei or > 2 lymphocytic nuclei [3,4]. The lymphoma exhibits morphologic variants denominated as ~immunoblastic variant wherein neoplastic cells delineate a singular, centric nucleolus ~Centro plastic variant wherein neoplastic cells exhibit two to four nucleoli ~anaplastic variant wherein neoplastic cells are imbued with anaplastic nuclei. Cellular component may simulate anaplastic large cell lymphoma or Reed-Sternberg cells [3,4] (Figures 1 & 2). Lugano classification of staging non-Hodgkin’s lymphoma is denominated as
Figure 1: Diffuse large B cell lymphoma depicting effaced lymph node architecture with diffuse dissemination of large, atypical lymphoid cells with vesicular chromatin and prominent nucleoli.
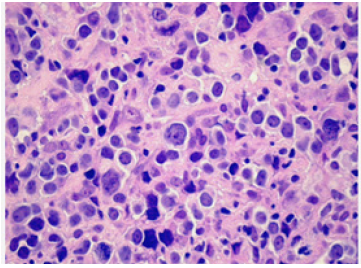
Figure 2: Diffuse large B cell lymphoma delineating scattered, large atypical lymphoid cells with vesicular nuclear chromatin and prominent nucleoli.
A. Stage I is described as ~lymphoma confined to singular
lymph node region (I)~lymphoma incriminates singular extralymphatic
organ or extra-nodal site whereas lymph node
involvement is absent (IE)
B. Stage II is described as ~lymphoma incriminating ≥2
lymph node regions on one side of diaphragm (II) ~lymphoma
incriminates singular organ and regional lymph nodes along
with or absence of neoplasm confined to diverse lymph node
regions on one side of diaphragm (IIE)
C. Stage III where lymphoma incriminates lymph node
regions on opposite sides of diaphragm (III)
D. Stage IV where lymphoma disseminates to diverse extralymphatic
organs as bone marrow, hepatic or pulmonary
parenchyma along with or devoid of incrimination of various
lymph node groups (IV) [4,5].
Progressive disease or refractory non-Hodgkin’s lymphoma is described as tumour expansion or dissemination during course of therapy of initially detected lymphoma. Recurrent or relapsed non- Hodgkin’s lymphoma is described as lymphoma which reappears following treatment. Reoccurrence may ensue within zone of initial incrimination or within diverse body sites. Reoccurring lymphoma may appear immediately following commencement of initial therapy or within decades. Recurrent lymphoma mandates repetitive staging or ‘re-staging’, contingent to pertinent staging system.
Diffuse large B cell lymphoma (NOS) is immune reactive to B cell markers as CD20, PAX5, CD79a, CD19 or CD22. Subtyping for cell of origin manifests immune reactive CD10, MUM1 and BCL6. Double expresser or ‘double hit’ neoplasms are immune reactive to BCL2 and MYC. Besides, lymphoma may be immune reactive to CD30 and CD5. Ki67 proliferative index is elevated. Epstein Barr Encoding Region (EBER+) is discernible upon In Situ Hybridization (ISH) [6,7]. Diffuse large B cell lymphoma (NOS) is immune nonreactive to CD3, cyclin D1 or CD5, Terminal Deoxy-nucleotides Transferase (TdT) or Anaplastic Lymphoma Kinase (ALK) [6,7]. Subtyping of cell of origin with immunohistochemistry (staining within ≥30% neoplastic cells) is designated as
A. Germinal Centre B Cell (GCB) subtype depicts CD10+ or
CD10-/BCL6+/MUM1- immune phenotype.
B. Non-Germinal Centre B Cell (GCB) subtype composed of
activated B cell (ABC) variant and unclassifiable neoplasms
as discerned with Gene Expression Profiling (GEP) delineates
CD10-/ BCL6- or CD10-/BCL6+/MUM1+ immune phenotype.
C. Double expresser status configures ~30% of diffuse large
B cell lymphoma (NOS) and exhibits immune reactive BCL2 (>
50% tumour cells) and MYC (> 40% tumour cells) along with an
absence of concordant chromosomal rearrangements (double
expresser). Aforesaid lymphoma is associated enhanced
possible tumour reoccurrence into Central Nervous System
(CNS) and inferior survival.
D. Double hit’ diffuse large B cell lymphoma is associated with
genetic rearrangements of MYC and BCL2 along with or devoid
of BCL6 as detected by Fluorescent In Situ Hybridization (FISH)
and is categorized as high-grade B cell lymphoma. Upon flow
cytometry, a population of enlarged, monoclonal B cells with
enhanced forward scatter is observed [7,8]. Gene Expression
Profiling (GEP) can suitably identify molecular subtypes of
diffuse large cell B cell lymphoma (NOS) as Activated B Cell
(ABC) and Germinal Centre B cell (GCB) variants. Alternatively,
cogent immunohistochemistry may appropriately discern
molecular subtypes [7,8].
E. Fluorescent In Situ Hybridization (FISH) is employed to
differentiate DLBCL(NOS) from high grade B cell lymphoma
demonstrating MYC and BCL2 and/or BCL6 genetic
rearrangements ~BCL2 genetic rearrangement (~30%) is
associated with activated B cell (<5%) and germinal centre
B cell (~50%) categories ~BCL6 genetic rearrangement
(~30%) is exemplified within activated B cell (~30%) and
germinal centre B cell (~15%) ~MYC rearrangement (~14%)
is encountered within activated B cell and germinal centre B
cell. Nearly ~50% lymphomas depict concordant BCL2 or BCL6
genomic rearrangements.
F. Polymerase Chain Reaction (PCR) is adopted for
evaluating B cell genetic rearrangements concurrent with
clonal immunoglobulin genetic rearrangements.
G. Diffuse large B cell lymphoma is endowed with
heterogeneous mutational manifestations demonstrating
repetitive alterations within ≥150 genes [7,8]. Frequent genetic
mutations appear within KMT2D, MYD88, PIM1, CREBBP,
HIST1H1E, BCL2, SPEN, ARID1A or TP53 genes.
Commonly observed genomic variants of diffuse large B cell lymphoma occur as ~MYD88 L265P and CD79B genetic mutation with preponderant activated B cell subtype, gain within chromosome 18q, akin to primary extra-nodal lymphomas and an inferior survival. ~BCL6 genomic translocation and chromosomal mutation of NOTCH2 accompanied with predominant activated B cell subtype with B cell receptor signalling, genetically simulating marginal zone lymphoma delineates superior survival. ~BCL2 genomic translocation and chromosomal mutation within EZH2 or various chromatin modifiers along with germinal centre B cell subtype simulate genetic features of B cell lymphomas of germinal centre origin.
Diffuse large cell B cell lymphoma (NOS) requires segregation from neoplasms such as high grade B cell lymphoma with MYC and BCL2 or BCL6 rearrangements, blastoid and pleomorphic variants of mantle cell lymphoma, Burkitt’s lymphoma, grade III follicular lymphoma, diffuse large cell B cell lymphoma immune reactive to Epstein Barr virus (EBV), muco-cutaneous ulcer immune reactive to EBV, primary mediastinal (thymic) large B cell lymphoma, T cell or histiocyte rich large B cell lymphoma, primary Central Nervous System (CNS) lymphoma, intravascular large B cell lymphoma, primary cutaneous DLBCL leg type, lymphomatoid granulomatosis, DLBCL associated with chronic inflammation, DLBLC (NOS) immune reactive to Human Herpes Virus 8(HHV8) or large B cell lymphoma immune reactive to Anaplastic Lymphoma Kinase (ALK). Diffuse large B cell lymphoma (NOS) can be appropriately discerned with cogent clinical history, morphological evaluation, immuno-phenotyping, and cytogenetic assay as Fluorescent in Situ Hybridization (FISH).
Around 50% instances demonstrate elevated levels of Lactate Dehydrogenase (LDH). Diffuse large B cell lymphoma (NOS) can be suitably treated with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone). Besides, diverse regimens incorporating rituximab can be employed [7,8]. Factors contributing to inferior prognostic outcomes are described as
A. Clinical factors as increasing age, elevated levels of LDH,
extra-nodal lymphoma, bulky disease, Ann Arbor stage III
or stage IV or instances demonstrating Eastern Cooperative
Oncology Group (ECOG) performance status ≥2.
B. Cell of origin designated as ~activated B cell subtype
(~40%) delineates proportionate 5-year progression free
survival at ~50% and germinal centre B cell subtype (~60%)
exhibits proportionate 5-year progression free survival at ~
80%.
C. Chromosomal translocation of MYC gene.
D. Double expresser status enunciates
immunohistochemistry concordant with MYC (≥ 40% tumour
cells) and BCL2 (≥ 50% tumour cells) along with or absence of
concurrent genetic rearrangements of MYC and BCL2.
E. Bone marrow infiltration is associated with enhanced
possibility of reoccurring central nervous system disease. Stage
III and stage IV lymphomas are commonly discerned and can
be appropriately treated and alleviated, contingent to subtype
of non-Hodgkin’s lymphoma. Therapeutic strategies and
prognostic outcomes of stage III and stage IV are identical.
References
- Ames A, Lee D (2022) Updates in the diffuse large b-cell lymphoma treatment landscape. J Adv Pract Oncol 13(3): 341-344.
- Padala SA, Kallam A (2022) Diffuse large B cell lymphoma. Stat Pearls International, Treasure Island, Florida, USA.
- Minghan Q, Shan W, Chen X, Wang H (2022) Update on diffuse large B-cell lymphoma: Highlights from the 2022 ASCO annual meeting. Cancer Biol Med 19(8): 1117-1120.
- Susanibar AS, Barta SK (2021) Update on diffuse large b cell lymphoma: A review of current data and potential applications on risk stratification and management. Am J Hematol 96(5): 617-629.
- Loeffler WH, Kreuz M (2022) Classifying germinal center derived lymphomas-navigate a complex transcriptional landscape. Cancers (Basel) 14(14): 3434.
- Kim E, Jiang Y, Tao X, Alexander B, Andrea K, et al. (2022) Prognostic mutational subtyping in de novo diffuse large B-cell lymphoma. BMC Cancer 22(1): 231.
- Chapuy B, Stewart C, Andrew J, Jaegil K, Atanas K (2018) Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med 24(5): 679-690.
- Atallah SA, Robertson MJ (2022) Novel Immune-based treatments for diffuse large b-cell lymphoma: The post-CAR T cell era. Front Immunol 13: 901365.
© 2023 Anubha Bajaj. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






