- Submissions

Full Text
Developments in Clinical & Medical Pathology
Is the BD Barricor Tube the New Standard for Lithium Heparin Plasma?
Goce Dimeski * and Julie Johnston
Department of Chemical Pathology, University of Queensland, Australia
*Corresponding author: Goce Dimeski, Chemical Pathology, University of Queensland, Princess Alexandra Hospital, Ipswich Road, Woolloongabba, 4102, Australia
Submission: May 17, 2018;Published: June 07, 2018

ISSN:2690-9731 Volume1 Issue3
Abstract
Background: Lithium heparin plasma from gel separator tubes has one major advantage over serum it can be centrifuged without delay. The major disadvantage is it contains a large number of cells, cell stroma and fibrin which renders it unsuitable for delayed testing of some analytes e.g. potassium.
Methods: BD tubes, plasma separator tube - PST, Barricor tube, rapid serum tube - RST and serum separator tube-SST were evaluated for stability of potassium, glucose, LDH and phosphate at different storage time points and centrifugation settings from 22 participants. Additionally tubes from a participant were centrifuged at 3000g, and the buffy coat containing sample was then transferred into a Cytospin centrifuge to prepare slides for staining and content examination.
Results: The Barricor tube showed decreased changes in analyte concentrations compared with the PST tube. The stability of analytes was best in the RST and at 3000g across all tubes. The buffy coat study showed the PST tube had the largest number cells present.
Conclusion: The Barricor tube offers improvement in lithium heparin plasma quality due to reduced cell numbers leading to decreasing the change in potassium, LDH and phosphate results from cellular lysis and glucose results from cellular consumption upon sample storage. Based on the data from this study the Barricor tube should become the new standard for lithium heparin plasma.
Keywords: Barricor; Tubes; Plasma; Serum; Gel separator
Introduction
Biochemical analysis primarily uses lithium heparin plasma or serum specimens, with serum being the more commonly used specimen type. It is well documented the lithium heparin and serum tubes do not always produce the optimal quality sample to meet timeliness and accuracy requirements, and the deficiency of these tubes are well known [1-3]. Obtaining the highest quality sample is in large part dependent on the tube components (procoagulant/anticoagulant, surfactant and gel separator) [4] and centrifugation settings [5]. Gel separators offer greater stability of analytes, by separating cells from plasma or serum and almost eliminating subsequent changes in analytes due to cellular activity or release from cellular rupture post centrifugation. The presence of “white particulate matter” on top of the gel consisting of cells and fibrinogen in lithium heparin impacts on LDH, and there is presence of oil droplet which was first reported from our laboratory in 2004 [6]. Cadamuro et al. [7] observed the presence of fat droplets in the “white particulate matter” that most probably originated from the separator gel in the tubes. Gel separator tubes have been reported to affect measurements of therapeutic drugs [8], red blood cells surpassing the separator gel barrier in both plasma and serum tubes increasing the potassium concentration [9], and the separator gel components in tubes has been the source of interference in their liquid chromatography-MS testosterone assay [10].
BD released the next-generation blood separation technology lithium heparin tube, Barricor designed to enhance plasma quality by lowering cell count in plasma. Rather than using gel as the separator it uses a mechanical device, high density plastic to separate cells from plasma. There are only a few publications on the Barricor tube and one is by Füzéry et al. [11] for suitability with the Beckman TnI assay. In this study we examined the effect on cell count in the Barricor tubes by using four biochemical analytes (potassium, glucose, phosphate, and LDH) and examining the composition of the buffy coat.
Materials and Methods
The study was in two parts. The study compared the BD Barricor tube (#365032, Lot 6011667) against the current commercial tubes from BD lithium heparin tube or plasma separator tube (PST) (#367375, Lot 6179937), rapid serum tube (RST) (#368774, Lot 160308), and the serum separator tube (SST) #367954, Lot 6144963). Part one was a comparison of the most significantly affected common analytes (potassium, glucose, phosphate, and LDH) by the presence of cells in the plasma or serum component post centrifugation and gel/mechanical device separation at four time points. In total 22 healthy participants were recruited with appropriate ethical approval and informed consent. The blood was collected by an experienced phlebotomist into serum tubes first (RST or/and SST) then into the lithium heparin tubes (PST or/and Barricor). The RST, PST and Barricor tubes were mixed as recommended by BD and centrifuged without delay while the SST tube was allowed to clot for 30 minutes then centrifuged. All tubes were centrifuged at 4000, 3000 and 2050g for 10 minutes at 20 °C. The 2050g was selected as per BD recommendations [12]. The samples were analysed at 0 time, 24, 48 and 168 hours post storage at 2-8 °C. Analysis was performed within ~30 minutes post centrifugation on a DxC800 general chemistry analyser (Beckman Coulter, Brea, CA, USA). Results were significant and analytically important when the percent difference was greater than the upper limit of the cumulative imprecision of the between-run coefficient of variations (CVs) from the internal quality control concentrations on the Beckman analysers. The acceptable cut-off CVs used was: <2% for potassium at 3.84mmol/L, <3% for glucose at 4.5mmol/L, <2.5% for LD at 182 U/L and <2% for phosphate at 1.01mmol/L. These acceptable limits are significantly lower than the withinsubject coefficient of variation published by Ricos et al. [13] <4.8% for potassium, <6.5% for glucose, <6.6% for LD, and <8.5% for phosphate in serum. The second part was to examine the buffy coat in each of the four tubes and demonstrate the cell presence and how this aligns to analytical changes. This was performed from the blood of a single participant. The blood was collected in each of the four tubes and then the tubes were centrifuged at 3000g post completion of part one of the studies where 3000 g showed the least changes and aligns with previous findings [5]. Post centrifugation, all but the last ~0.5mL of plasma or serum was left in each tube. The 0.5mL sample in each tube was well mixed to obtain a homogenous mixture, and then a 0.1mL aliquot was transferred into the cytospin cups with slides (Cytospin 4, Thermo Fisher Scientific, Sydney, Australia) to concentrate the cells. The samples were centrifuged at 1260 rpm for 5 minutes. The slides were stained with May-Grunwald-Giemsa stain and processed as per normal blood film. The slides were examined for composition at x20 and x400 magnification. The cell count was performed on grid slides with counting chambers using a phase contrast microscope (x40 magnification). The concentration of all cell is x106. The samples were diluted 1 in 20 with normal saline prior to loading on the counting chamber.
Results
The study was in two parts. The study compared the BD Barricor tube (#365032, Lot 6011667) against the current commercial tubes from BD lithium heparin tube or plasma separator tube (PST) (#367375, Lot 6179937), rapid serum tube (RST) (#368774, Lot 160308), and the serum separator tube (SST) #367954, Lot 6144963). Part one was a comparison of the most significantly affected common analytes (potassium, glucose, phosphate, and LDH) by the presence of cells in the plasma or serum component post centrifugation and gel/mechanical device separation at four time points. In total 22 healthy participants were recruited with appropriate ethical approval and informed consent. The blood was collected by an experienced phlebotomist into serum tubes first (RST or/and SST) then into the lithium heparin tubes (PST or/and Barricor). The RST, PST and Barricor tubes were mixed as recommended by BD and centrifuged without delay while the SST tube was allowed to clot for 30 minutes then centrifuged. All tubes were centrifuged at 4000, 3000 and 2050g for 10 minutes at 20 °C. The 2050g was selected as per BD recommendations [12]. The samples were analysed at 0 time, 24, 48 and 168 hours post storage at 2-8 °C. Analysis was performed within ~30 minutes post centrifugation on a DxC800 general chemistry analyser (Beckman Coulter, Brea, CA, USA). Results were significant and analytically important when the percent difference was greater than the upper limit of the cumulative imprecision of the between-run coefficient of variations (CVs) from the internal quality control concentrations on the Beckman analysers. The acceptable cut-off CVs used was: <2% for potassium at 3.84mmol/L, <3% for glucose at 4.5mmol/L, <2.5% for LD at 182 U/L and <2% for phosphate at 1.01mmol/L. These acceptable limits are significantly lower than the withinsubject coefficient of variation published by Ricos et al. [13] <4.8% for potassium, <6.5% for glucose, <6.6% for LD, and <8.5% for phosphate in serum. The second part was to examine the buffy coat in each of the four tubes and demonstrate the cell presence and how this aligns to analytical changes. This was performed from the blood of a single participant. The blood was collected in each of the four tubes and then the tubes were centrifuged at 3000g post completion of part one of the studies where 3000 g showed the least changes and aligns with previous findings [5]. Post centrifugation, all but the last ~0.5mL of plasma or serum was left in each tube. The 0.5mL sample in each tube was well mixed to obtain a homogenous mixture, and then a 0.1mL aliquot was transferred into the cytospin cups with slides (Cytospin 4, Thermo Fisher Scientific, Sydney, Australia) to concentrate the cells. The samples were centrifuged at 1260 rpm for 5 minutes. The slides were stained with May-Grunwald-Giemsa stain and processed as per normal blood film. The slides were examined for composition at x20 and x400 magnification. The cell count was performed on grid slides with counting chambers using a phase contrast microscope (x40 magnification). The concentration of all cell is x106. The samples were diluted 1 in 20 with normal saline prior to loading on the counting chamber.
Figure 1: Stained slides from each of the BD tubes buffy coat layers to demonstrate visually the cell type and amount of cells present at 20x and 400x magnification.
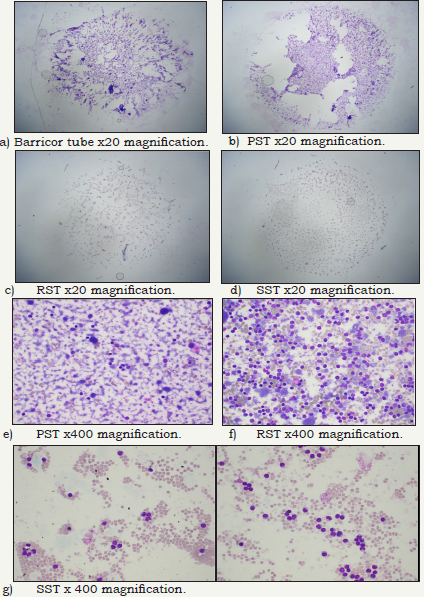
Table 1: Potassium, glucose, LDH and phosphate results using the different BD tubes at different centrifugation speeds and storage times. (# clinically significant difference).
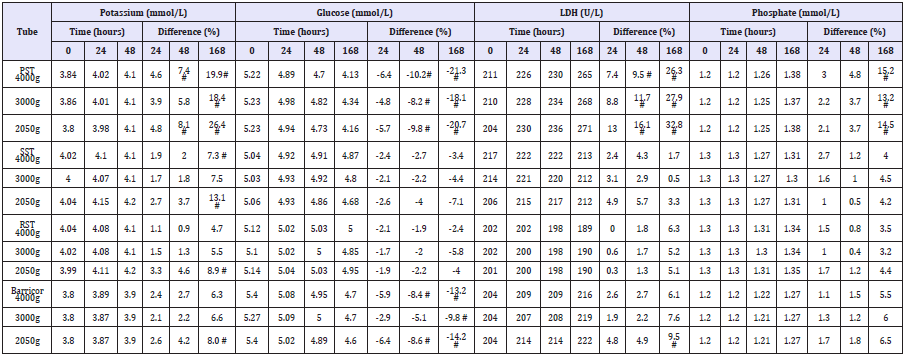
Table 2: Cell count of the buffy coat in each of the four tubes performed at x40 magnification with phase contrast microscope.
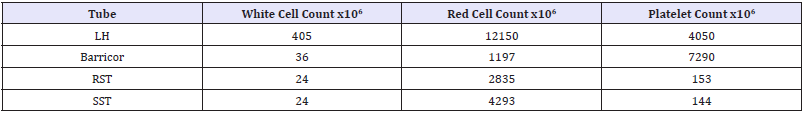
The biochemical analytes comparative stability results (means, percentage differences and if the difference is clinically significant) are shown in (Table 1). The Barricor tube plasma results clearly showed decreased changes in the biochemical analytes concentration over time when compared with the PST plasma. The PST plasma for all four analytes showed the most significant changes across all tubes, centrifugation settings and storage time points. It exceeded the allowable technical limits for each time point and centrifugation setting. The most significant changes across all tubes were observed with the 2050g centrifugation. The least changes observed overall were with the 3000g centrifugation, and with the serum from the RST. The slides, Figure 1 showed significant higher number of cells were present in the lithium heparin tubes, PST having higher red cells and white cells numbers than the Barricor tube which had slightly higher platelet numbers. The least number of cells were present in the RST serum sample. The breakdown of cell types from counts under microscope in each tube is shown in (Table 2). The haemolysis index levels measured on the Beckman Dx800 analysers were insignificant in all tubes, below levels that affect any analytes.
Discussion
Sample quality can influence accuracy of results of any test. The data from part one showed the mechanical barrier in the Barricor tube is significantly more effective in separating cells from the plasma than the gel in the PST. Hence analytical changes in the four analytes are minimised across the different storage time points and centrifugation settings. It is well known blood cells (red, white and platelets) are progressively lysed post storage, leading to release of potassium, LDH, and phosphate while viable cells continue to consume glucose [7]. The LDH activity decrease in serum tubes is due to fraction 4 (F4) instability on storage at 0-4 °C. The concentration of F4 falls rapidly in the first week, and disappears within 3 weeks [14]. Yet IFCC states serum is the preferred sample for LDH [15]. The data in this study showed heparin inhibits this degradation of the F4, hence LDH activity follows the same path as the other cell present analytes (potassium and phosphate), increasing with time. The 3000g centrifugation setting was the most effective in minimising cell numbers presence in both plasma and serum, and provided the best stability in concentration of analytes. The slides in part two data clearly showed the amount of buffy coat varies from tube to tube. With the x20 magnification the red and white cell numbers were highest in the PST, followed by the Barricor tube, the SST and the least in the RST tube. The Barricor tube had the highest platelet cell count. The x400 magnification provided clear distinction on the cell types present, and that the platelets were removed during the clotting process in the RST and SST tubes. WHO report that a buffy coat presents with all three cell types, cell stroma plus a lot of fibrin [16]. This is only true for lithium heparin plasma. If the plasma specimen is mixed (e.g. during sub-sampling or handling), it will become turbid because of disturbance of the buffy coat, re-suspension of cell-containing material along with the fibrin, decreasing the specimen integrity.
The ‘white particulate matter” formation of insoluble fibrin is enhanced when heparin plasma is stored at low temperature [17]. It has also been suggested that individual molecules within pharmaceutical heparin preparations may have procoagulant activity particularly in the absence of antithrombin III [18]. Stimulation of platelet factor 4 (PL4), which is a protein stored in the alpha granules of platelets that is released during platelet aggregation, can neutralize heparin [19,20]. PL4 may be released at the site of collection and into the sample or from platelets trapped in microclots which in turn can aid the formation of fibrin strands in the specimen. If all of these physico-chemical factors are present in the right balance, fibrin formation is likely to be enhanced in heparinised specimens. Over time heparin activity in stored lithium heparin blood specimens is decreased, mainly due to PL4 activity. In our tertiary level laboratory up to 5% of the lithium heparin specimens stored for two days at 4 °C showed visible fibrin formation which would render them unsuitable for re-analysis. Such stored plasma specimens require aliquoting and re-centrifugation for re-testing or performing additional tests and due to the presence of cells renders the plasma samples unsuitable for determination of some analytes such as potassium and glucose. The only other notable factor is the Barricor tube with the mechanical separator leads to slightly decreased plasma volume. The limitations of this study are therapeutic drugs and broader range of analytes was not studied for stability due to the mechanical separator, if it does bind to drugs leading to concentration changes.
Conclusion
The Barricor tube offers improvement in lithium heparin plasma quality as shown by the decreased changes in potassium, LDH and phosphate concentrations due to cellular lysis and glucose results from cellular consumption upon sample storage. The least differences in results were observed across all four tubes with 3000g centrifugation speed. Based on the data from this study the Barricor tube should become the new standard for lithium heparin plasma.
Acknowledgement
BD provided the Barricor and RST tubes but played no part in study design or write up.
References
- Dimeski G, Masci PP, Trabi M, Lavin MF, De Jersey J (2010) Evaluation of the becton-dickinson rapid serum tube: does it provide a suitable alternative to lithium heparin plasma tubes? Clin Chem Lab Med 48(5): 651-657.
- Dimeski G, Johnson J, Masci PP, Zhao KN, Brown N (2017) Evaluation of the greiner bio-one serum separator BCA fast tube. Clin Chem Lab Med 55(8): 1135-1141.
- Yan R, Lou A, Watts G, Tarr H, Smith H, et al. (2014) Comparison of becton dickinson vacutainer rapid serum tube with the serum separator tube for routine chemistry and immunoassay tests. J Clin Pathol 67(7): 599-604.
- Bowen RA, Adcock DM (2016) Blood collection tubes as medical devices: The potential to affect assays and proposed verification and validation processes for the clinical laboratory. Clin Biochem 49(18): 1321-1330.
- Dimeski G, Solano C, Petroff MK, Hynd M (2011) Centrifugation protocols: tests to determine optimal lithium heparin and citrate plasma sample quality. Ann Clin Biochem 48(3): 218-222.
- Dimeski G, Badrick T, Flatman R, Ormiston B (2004) Roche IFCC methods for lactate dehydrogenase tested for duplicate errors with greiner and becton-dickinson lithium-heparin and Greiner serum samples. Clin Chem 50(12): 2391-2392.
- Cadamuro J, Wiedemann H, Felder TK, Mrazek C, Kipman U, et al. (2017) What´s floating on my plasma? Biochem Med (Zagreb) 27(2): 430-433.
- O’Keane MP, Cunningham SK (2006) Evaluation of three different specimen types (serum, plasma lithium heparin and serum separator gel) for analysis of certain analytes: clinical significance of differences in results and efficiency in use. Clin Chem Lab Med 44(5): 662-668.
- Babic N, Zibrat S, Gordon IO, Lee CC, Yeo KT (2012) Effect of blood collection tubes on the incidence of artefactual hyperkalemia on patient samples from an outreach clinic. Clin Chim Acta 413(19-20): 1454-1458.
- Shi RZ, Van Rossum HH, Bowen RA (2012) Serum testosterone quantification by liquid chromatography-tandem mass spectrometry: Interference from blood collection tubes. Clin Biochem 45(18): 1706- 1709.
- Füzéry AK, Raizman JE, Goudreau BL, Moses K, Niemann K, et al. (2017) The BD Barricor blood collection tube is an acceptable and robust alternative to the PST for use with the Beckman AccuTnI+3 assay. Clin Biochem 50(15): 851-857.
- BD Vacutainer BarricorTM Lithium HeparinN Blood Collection Tubes.
- Ricos C, Alvarez V, Cava F, Garcia-Lario JV, Hernandez A, et al. (1999) Current databases on biological variation: Pros and cons and progress. Scand J Clin Lab Invest 59(7): 491-500.
- Kreutzer HH, Fennis WHS (1964) Lactic dehydrogenase isoenzymes in blood serum after storage at different temperatures. Clin Chim Acta 9(1): 64-68.
- IFCC (1994) IFCC’s Committee on enzymes, Approved recommendation on IFCC methods for the measurement of catalytic concentration of enzymes. EUR J Clin Chem Clin Biochem 32(8): 639-655.
- World Health Organisation (2002) Use of anticoagulants in diagnostic laboratory: stability of blood, plasma and serum samples. Geneva, Switzerland.
- Cameron CL, Fisslthaler B, Sherman A, Reddigari S, Silverberg M (1989) Studies on contact activation: effects of surface and inhibitors. Med Prog Technol 15(1-2): 53-62.
- Smith SA, Morrissey JH (2008) Heparin is procoagulant in the absence of antithrombin. Thromb Haemost 100(1): 160-162.
- Bates SM, Weitz JI (2000) The mechanism of action of thrombin inhibitors. J Invasive Cardiol 1(2): 27-32.
- Levy JH, Cormack JG, Morales A (1995) Heparin neutralization by recombinant platelet factor 4 and protamine. Anesth Analg 81(1): 35-37.
© 2018 Goce Dimeski. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






