- Submissions

Full Text
Developments in Anaesthetics & Pain Management
Pain in Individuals with Alzheimer’s Disease and Other Dementia-Related Diseases: Too Often Overlooked
Marnin Joseph Romm*
School of Health Professions, University of Texas Medical Branch, USA
*Corresponding author: Marnin Joseph Romm, School of Health Professions, University of Texas Medical Branch, USA
Submission:February 24, 2023;Published: March 09, 2023

ISSN: 2640-9399 Volume2 Issue4
Abstract
Dementia is a core symptom in Alzheimer’s Disease (AD) and Other Dementia Related Diseases (ODRDs). It accounts for one of the foremost health concerns in elderly individuals with ongoing decline in cognition, activities of daily living and behavior. In addition, what is commonly not accounted for as a primary symptom in AD and ODRDs is pain. Unfortunately, Chronic Pain (CP) is most often overlooked and therefore underdiagnosed due to insufficient knowledge around the CP neuroscientific mechanisms in AD and ODRDs. In turn, a thorough biopsychosocial assessment of pain in this population group does most often not occur and therefore, as a consequence, necessary pain management is not implemented. There is still insufficient tools or a battery of instruments that are fully reliable and valid to you use when assessing pain in AD and ODRDs for both communicative and non-communicative individuals. Furthermore, the instruments that do exist, are not validated for use in a sufficient number of global settings. Interdisciplinary pain management programs still need to be further advocated for when managing CP in patients with AD and ODRDs. Both pharmacological, interventional, and non-pharmacological treatment modalities to manage pain is most often necessary to target all the pain pathophysiological mechanisms that produce an individual’s pain experience. Thus, a multitude of treatments should be used to focus on the impact that CP has on the lives of people living with AD and ODRDs. Of utmost importance is maintaining a patient-centered individualized treatment approach. This review aims to reveal what is currently in practice in terms of assessing and managing pain in AD and ODRDs, and the current gaps that are still in place, that require further rigid research to translate into stronger evidence based-clinical practice.
Keywords: Chronic Pain (CP); Alzheimer’s Disease (AD); Other Dementia Related Diseases (ODRDs); Pain assessment; Interdisciplinary pain management; Biopsychosocial framework
Introduction
The incidence of neurodegenerative diseases is going to rise in correspondence to life span increasing [1]. In turn, with an increasingly aging population moving forward, there will be an expanding number of people who will suffer from pain as well as from conditions that entail symptoms of dementia [2]. Over the past number of years, palliative care has become further involved in the management of people with neurodegenerative conditions [3]. The most common neurodegenerative diseases generally occur later in life and are largely accompanied by increasing motor and cognitive deficiencies [1]. These impairments are managed in conditions such as Alzheimer’s Disease (AD) and Other Dementia-Related Diseases (ODRDs), Parkinson’s Disease (PD) and illnesses related to PD, Motor Neuron Disease (MND), Huntington’s Disease (HD), Spinocerebellar Ataxia (SCA), Multiple Sclerosis (MS), Spinal Muscular Atrophy (SMA) and other neurodegenerative diseases. Dementia accounts for one of the foremost health concerns in elderly individuals with ongoing decline in cognition, activities of daily living and behavior, that all together may lead to disability [4]. Globally, approximately 40 million people over the age of 65 suffer from dementia, and 70% of these individuals are affected by AD [4]. The United States (US) has the second largest population of people with dementia, being approximately 4 million [5]. The symptom of pain is a frequent complaint by many patients who experience these and other neurodegenerative diseases.
The prevalence of pain in adults over 65 years of age in the US is variable, however there are reports suggesting the rates of pain in geriatric populations are around 21% (4 million people); notably, not all these individuals have AD or ODRDs [5]. Importantly, the majority of this pain is classified as persistent pain/Chronic Pain (CP) [5]. In the last approximately 10 years, pain and dementia has drawn in surmountable interest, because of the notion that pain is not picked up by clinicians and therefore undertreated in patients with dementia [6,7]. For clarification, dementia is not a specific, but rather a general umbrella term for decline in mental and other symptoms caused by pathologies impacting upon the brain [8,9]. AD is known to be the most common cause of dementia, affecting at least 27 million people worldwide, which accounts for 60-70% of global dementia cases [4,5]. In addition, notably, there are many types of dementia that exist [3,4]. There are an estimated 35 million people globally with dementia of whom approximately 50% experience frequent pain [10]. For the purposes of this review, the focus is directed towards AD and ODRDs. Individuals with AD and ODRDs, as well as those with the other diseases mentioned above, who experience pain, appear to not receive the necessary assessment and treatment of pain across a broad range of clinical environments [11]. Consequently, precise treatment protocols for the various conditions often don’t consider pain as a core symptom, and therefore the management of pain is commonly left solely in the discretion of the individual clinicians [1,3].
Pain is a complex perceptual and subjective experience that contains sensory, emotional/psychological and cognitive elements [12]. Neuropathological changes that occur in AD and ODRDs, are understood to be responsible for modifications in pain perception [13]. Pain is considered by many as one of the most incapacitating symptoms that patients with AD and ODRDs may experience. However, there appears to be a general lack of attention towards the pain management component when intervening with these individuals. The above may be the result of treating practitioners most often focusing on what is commonly understood to be the specific core illness symptoms [1]. Achterberg et al. [11] assert that patients with dementia appear to feel the intensity of pain and affective component of pain differently to individuals with intact cognitive functional abilities [11]. Furthermore, their decline in communicative abilities makes it even more difficult for clinicians to notice pain and thus fully assess and treat potential pain in these patients. However, an integrated care approach towards a large number of patients experiencing neurodegenerative conditions should involve vigorous efforts to assess and treat pain whenever necessary [3]. As with any patient with CP, the establishment of a comprehensive treatment plan is integral, and in the population of AD and ODRDs, specifically, an interdisciplinary approach is vital the development of a multimodal pain management plan [14]. A strong interdisciplinary style should include a thorough assessment, managing polypharmacy and pharmacotherapy, psychological evaluation and care, physical rehabilitation and other interventional procedures [15]. The team should always include the individual with dementia, their family, caregivers, and different members of the clinical team. This should ensure that a patient-centered approach is embraced, and includes the values, preferences and needs of the person with dementia [16].
The International Association for the Study of Pain (IASP 2020) definition of pain is: “An unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage”[17]. Importantly, whilst paying reference to the above definition of pain, pain is commonly expressed through various behavioral patterns such as agitation or withdrawal and not only through the means of verbal communication means. Pain is considered one of the most significant causes of Behavioral And Psychological Symptoms Of Dementia (BPSD) [18]. However, unfortunately, these behaviors in patients with AD or ODRDs, are often mistakenly perceived as psychiatric symptoms [11]. BPSD can be a consequence of pain most commonly in the form of agitation or aggression, which adds to stress levels of both patients and caregivers [11]. The underlying source of the pain in these disorders is often inconsistent though, and thus understanding the neuroscientific mechanisms underpinning the pain experience in patients with these conditions, is crucial to treat the symptoms effectively. Based on previous and current research, up until this point, as with the majority of CP conditions, the full determinants around the pathophysiological mechanisms of pain in neurodegenerative pathologies is still inconclusive [1].
Based on the outline above, the following review will particularly focus on pain in dementia-type disorders (referred to as ODRDs and AD). The evidence, as will be supported through the proceeding review, supports a high incidence of pain in AD and ODRDs. This highlights the need for neurologists and other healthcare clinicians working with patients diagnosed with AD and ODRDs, to become fully involved in managing their patients’ pain appropriately. This includes understanding CP pathophysiological mechanisms and making use of the correct biopsychosocial assessment/s tools and instruments to use when examining patients in their entirety (biological/physiological and anatomical, psychological/affective, and social components to their being). The assessment of pain is central to improving quality of life and even to reduce the risk of death in individuals with dementia [18]. The review will firstly provide an extremely diluted explanation around CP neuroscientific mechanisms at play with a navigation pointing towards pathophysiological mechanisms underlying pain in AD and ODRDs. Proceeding this short overview of CP physiological processes, the core focus of this review will then be executed; patient-centered biopsychosocial assessment of a patient with AD or ODRDs. Following the above, a very brief outline of management targeting the multifaceted mechanisms contributing to the experiences of CP in patients with AD or ODRDs, will then be succinctly described.
Pathophysiological mechanisms underlying pain in ad and dementias
The nociceptive flexion reflex as well as brain activity as a consequence of noxiously painful stimulation in research studies, have been found to be amplified in patients suffering from dementia [19,20]. Neuroimaging and neuropathological studies have both indicated interconnected brain regions that are crucial in the mediation of pain processing [21-30]. Kunz et al. [31] assert that there is surmountable evidence that dementia modifies the processing of pain [31]. Investigations into whether this alteration in pain processing the result of general cognitive decline is or rather due to specific entities relating to cognitive functioning [31]. The research concluded that there is a close relationship between executive functioning and pain receptiveness; neurodegeneration in the prefrontal cortexes does not only contribute to decreased executive functioning in patients with dementia but also to a reduction in pain inhibition thus making the patient more susceptible to pain perception [31]. The following section merely provides a context around CP mechanisms in general, specifically looking at the brain. This following brief outline is intended to assist with a simplified understanding of some of the pathophysiological mechanisms at play in patients who have AD and ODRDs experiencing CP. Although extremely simplified, Achterberg et al, alongside surmountable other researchers [11,28], have made note of two neuronal networks that are suggested to be most vital in the above pain processing system. Interested readers may want to consult the surmountable amounts of literature that dissects neurophysiological pain mechanisms in greater details.
The neuropathological changes that generally take place in AD selectively impact crucial areas concerning the medial pathway of pain [12]. The medial pain system, the most complex, is made up of the amygdala, medial thalamus, hippocampus, Anterior Cingulate Cortex (ACC) and prefrontal cortex [8,22]. This system includes the spinothalamic tracts, the spinoreticular tract and the spinomesencephalic tract [32]. This pathway is responsible for the cognitive-evaluative and motivational-affective dimensions of pain, as well as memory related to pain experiences [8,22]. Furthermore, autonomic-neuroendocrine facets are also facilitated by the medial system [11]. The more primitive sensory-discriminative aspects of pain (intensity, localization and quality of pain) are facilitated by the lateral pain system, which is suggested to be simpler than the medial pain system [8,22]. This system is made of, including others, the primary somatosensory areas (S1) and the lateral thalamic nuclei [11]. Like the medial system, the lateral system transmits messages regarding sensory-discriminative information through the spinothalamic tracts, however to the lateral thalamic nuclei [32]. From here, the most critical projections are suggested to be to the S1, insula and parietal operculum [32].
The integrity underlying the structure of the lateral pain system, regulates whether the pain threshold will be normal, in addition to the individual being able to identify qualities of the pain experience such as location of the pain, pain intensity and type of pain [33,34]. It is important to note, that these systems within the lateral pain system and the structures that the lateral pain system project to are typically protected in individuals with AD [35], in contrast to the structures and projections in the medial pain system. Therefore, it is suggested that pain perception, intensity and pain threshold are generally not affected in patients with AD [35]. Recently, a third pathway has been suggested that governs other crucial aspects of pain; the limbic pain system which controls the behavioral and affective aspects of pain [30,36]. Agitated behavior as a consequence of pain, as mentioned previously, may be a result of this third system being stimulated [36]. Table 1 effectively summarizes the important cortical regions and their function with respect to CP perception [32].
Table 1: Key cortical and brainstem structures and their roles in pain perception.
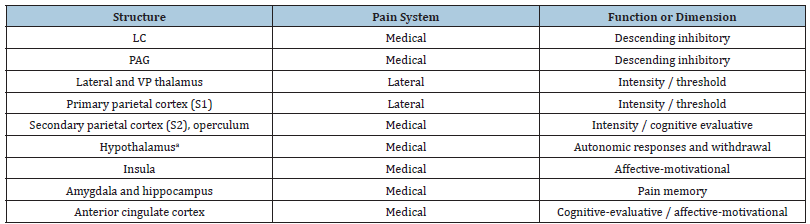
LC- Locus Coeruleus; PAG- Periaqueductal Grey; a-Mammillary bodies and paraventricular nuclei
Last but not least, autonomic responses as a result of pain may range from external signs such as pallor, perspiration, piloerection etc. to cardiovascular signs such as changes in heart rate or blood pressure to gastroenterological effects and/or urinary alterations [32]. These changes are largely navigated by the Periaqueductal Grey (PAG) [37], but most of all as a result of the function of the hypothalamus, which plays a large role in uncomfortable behaviors and autonomic and neuroendocrine consequences contributing to an individual’s pain perception [32]. The Tuberomammillary Nuclei (TN) (the only histaminergic nuclei in the brain) and the Paraventricular Nuclei (PN), are both nuclei housed within the connections between the hypothalamus, pontine reticular formation, prefrontal cortex, amygdala and hippocampus [37]. Normally, both the TN and PN produce various peptides that create an antinociceptive effect, however their function is changed in older individuals and in AD. Thus, this may further explain an increased pain response in individuals with AD [22,38]. It is still not fully clear which exact neuropathological changes are at play causing alterations in the perception of CP in patients with AD and ODRDs [6,11].
Even more so, there are many more answers still to be solidly identified in scientific literature around the potentially different mechanisms at play between AD, ODRDs and the different types of dementia (a detailed description regarding of types of dementia are not provided here, as this is not within the scope of this current review) [39]. What is known though, is that dementia is characterized by atrophy of cortical tissue, with various subtypes of dementia revealing different patterns of neurodegeneration [3,23]. However, with reference to the above, for some time there has still been much questioning around the perception of pain in patients with AD and possible disease related alterations to the Central Nervous System (CNS) transmission and processing of nociceptive input [40]. In a review by Lawn et al. [41], evidence is gathered explaining that pain processing is distorted in patients with AD and PD [41]. Cole et al. [19] directly looked at the above by means of psychophysical and fMRI measures [19]. Unlike what was described earlier on in this section with respect to the structures in the lateral pain system being maintained and therefore reducing the pain experience in individuals with AD, implementation of a noxious stimulus in the study by Cole et al. [19], revealed a mutual network of cortical activity in both the AD and control groups. Compared with the control group, the AD patients revealed heightened amplitude and duration of pain-related action in sensory, cognitive and affective processing cortical areas correlating to prolonged attention to the noxious stimulus [19]. Specifically, the prefrontal cortex is a key brain region involved in descending inhibition of pain and has been proven to show changes in activity in response to noxious stimuli in patients with AD [6,19]. In particular, in a study by Bunk et al. [6], it was found that there appears to be an association between a loss of grey matter in the medial orbitofrontal cortex (mOFC) and ACC, in addition to structural alterations in the white matter tracts connecting these prefrontal areas, with problems in the endogenous pain inhibitory system functioning in subjects who were cognitively compromised [6].
Thus, the idea that neurodegeneration in AD and ODRDs, in prefrontal areas, as well as in the descending pain modulatory system, might affect the processing of pain in these patients [6]. In turn, altered pain responses in patients with AD and ODRDs should be emphasized, as a consequence of the above [6], in addition to other sources. Therefore, of key note, pain perception and processing are not reduced in AD [19]. This once again emphasizes the amplified need for pain management in this population group [19]. However, it must be noted that depending on the progression and stage of the disease, the psychophysical results may differ due to the advancement of cognitive impairments. A thorough metaanalytical review has managed to identify numerous key biomarkers that are present in the Cerebrospinal Fluid (CSF) in patients with AD [10]. This identification of these core biomarkers in patients with AD show significant usefulness in clinical work, based on their capability to decrease misclassification levels when equated to the solitary application of clinical and/or neuropsychological evaluations of patients with potential AD [10,42]. Furthermore, this research revealed that the main biomarkers in CSF are ideal for differentiating patients with AD from patients who are healthy [10]. However, of importance, CSF biomarkers do not allow for the distinguishing between patients with AD and ODRDs [10], but more recent revised criteria for AD diagnosis includes CSF biomarkers in addition to neuroimaging biomarkers [43] as well as other critical biopsychosocial assessment criteria. Thus, the above once again indicates the necessity to implement a complex multifaceted and individualized assessment of all patients presenting with dementia-like symptoms to confirm accurate diagnostics. To add, based on the importance of this review, clinicians need to understand that these patients experience pain because of their complex pathophysiology, as described above. However, ignoring the key elements underlying psychosocial contributors towards the pain experience, would be detrimental to gaining a full picture into these patients’ pain perceptions and thus would not allow for thorough interdisciplinary pain management [44-48].
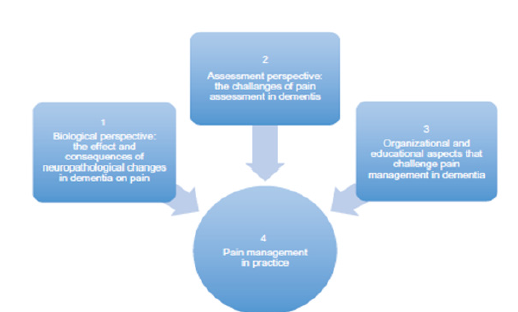
In a review by Cao et al. [49], they add to some of the pathological mechanisms underlying the link between AD, specifically, and CP. A dysfunction between the Locus Coeruleus (LC) and the Norepinephrine System (NE) may catalyze microglia activation, known to be triggered in patients with CP [46-48], may be the link between CP and AD [49]. Although CP induced LC-NE dysfunction may exacerbate AD pathogenesis via the proinflammatory immune system microglia, the pattern of the LCNA dysregulation in the patient with AD, as a comorbidity, is still not perfectly clear [49]. The key question is whether CP produces neuronal loss in the LC is still under much investigation, but it is certainly likely[50-55]. To summarize, the above section has provided an extremely diluted review of CP neuroscientific pain mechanisms, in general, however has attempted to paint a broad picture suggesting some of the complexities underlying the pain experience. Furthermore, a microscopic outline of only some of the multitude of neurophysiological and other mechanistic intricacies surrounding AD and ODRDs, with a focus on pain in these conditions has been provided. Figure 1, although not an all-inclusive figure as it excludes psychosocial elements at play in the development of the pain experience in these patients, further clarifies the overall challenges faced when treating pain in patients with symptoms of dementia. Therefore, paying attention to the above, with a specific focus on patients with dementia presenting in all neurodegenerative disease forms including AD, needs to be embraced. Therefore, the importance surrounding a thorough patient-centered pain assessment approach, as will be further discussed in the next section, and in turn an interprofessional pain management regimen, cannot be emphasized enough.
Figure 1:Challenges facing clinicians working with pain in patients with dementia [11].
Assessment of Alzheimer’s disease and other dementiarelated diseases
If pain is undertreated in the elderly, specifically in those with AD or ODRDs, it is largely due to derisory assessments, primarily relating to the presence of pain, but in addition the repercussions resulting from pain [32]. A full assessment should include a dive into all biopsychosocial factors impacting upon an individual’s pain experience. Therefore, an assessment should ideally include the patient at hand as well as family and caregivers to assist in supporting information when the patient with AD or ODRDs is unable to provide as such. At the very least, the following should be included in an assessment of a patient with AD or ODRDs experiencing pain: It is recommended that full and thorough narrative history taking of the patient’s pain is included. Starting with an all-inclusive narrative interview also allows the clinician to start building a strong therapeutic alliance with the patient [56-63]. Importantly, potential red flags should be ruled out as contributing to the individual’s pain [32]. A general and then neurological physical examination is necessary [32]. Pain intensity as well as location and pain interference measures should also be implemented. This should include functional, emotional and social consequences [32]. In addition autonomic repercussions should also be assessed [32], and in turn addressed.
The notion that pain may not be a highlighted symptom in patients with AD and ODRDs, is founded mainly on studies that have focused on non-verbal suggestions of pain, given that there is a general decrease in verbal communication associated with dementia and thus valid and reliable self-report measures of pain is difficult. Although debatable, it appears that experimental research has revealed that pain processing is not reduced in patients with ODRD’s and AD but rather increased [6]. Therefore, it is argued that pain in patients with dementia should be frequently assessed and accompanied by using tests that examine cognitive function, specifically examination of executive function [31]. Various cognitive entities such as memory, attention and executive function have been probed in order to try explain these changes in pain responses [1]. Tomasso et al. [1] highlight the need for greater use of quantitative assessment tools to examine the symptoms of pain in these neurodegenerative disorders [1], including AD and ODRDs. Researchers have thus developed pain instruments with varying degrees of intricacy that assess the different facets that make up the pain experience. As in previous research [19,20,64], the research by Bunk et al. [6] was unable to show differences in subjective responses to pain derived by physical pressure, between cognitively healthy and cognitively challenged individuals [6]. This was despite using a less complicated pain rating scale [6]. In addition, they could not show a statistically significant difference between groups in Conditioned Pain Modulation (CPM), when pain responses were evaluated by self-report measures [6].
As noted, CP is a complex perception and experience containing numerous factors, and therefore quantifying or measuring pain in an objective manner is an extremely difficult job [32]. Based on assessing pain through a biopsychosocial framework, specifically in patients with AD and ODRDs, aspects such as intensity, quality, location, affective burden, social impact, functional consequences and other variables associated with CP, are all challenges to adequately assess. Notably, communication and comprehension problems that are generally present in patients with AD and ODRDs, make the evaluation of the above factors extremely difficult to assess, with even greater challenges as the various diseases progress [32]. With reference to the definition of pain by the IASP (2020) [17], as outlined in the introduction section, it is critical to pay attention to the words “…resembling that associated with…”, as this key phrase indicates the importance to be able to assess pain not only through an individual’s ‘verbal communicative’ point of view but through other expressions of pain that do not include verbal ‘communication’. Thus, it is key to note that communication occurs through many different means besides ‘verbal communication’. The simplest pain visual scales such as the Visual Analogue Scale (VAS) or Wong Baker Face Rating Pain Scales, may be valid and reliable to use with patients who can ‘communicate’ and fully comprehend [32]. Assessing ‘communicative’ patients with AD and ODRDs, descriptive verbal scales, VASs, are generally useful [32], however these scales generally are very one-dimensional and merely generally examine pain intensity, which is a construct in CP that is not of the utmost importance in assessment and treatment. All other variables associated with an individual’s pain experience require far more multifaceted and time-consuming quantitative and qualitative procedures [32].
Many individuals who suffer from dementia present with ongoing deterioration in functional abilities, such that self-care is no longer feasible [65]. Along with this deterioration, digression in verbal communication often to a point where this type of communication is no longer present in the patient [65]. Thus, for those individuals less able to understand and communicate, it is necessary for clinicians to make use of more indirect signs for examining pain, such as expressive motor (body language, facial expressions) and/or autonomic signs [32]. Focusing ones attention on indirect signs for patients who are less communicative or noncommunicative with more advanced dementia, is of need in these patients’ assessments of pain [32]. Gonzalez et al. argue that such examinations require more complex instruments that measure the 3 facets of pain being intensity and location, emotional components and autonomic responses to pain [32]. However, this current review suggests that whether someone is communicative or not, these aspects of pain should always be assessed so to gain as full of a picture into the patients’ pain experiences rather than merely a ‘number’ quantifying their pain intensity. Although numerous scales are available that address the above 3 dimensions of pain, as suggested by Gonzalez et al. the instruments with the highest level of reliability, validity and consistency and therefore the most broadly excepted and recommended by experts in the field for use with this population group (patients that are non-communicative with AD or ODRDs) [66,67]. Examples of such instruments include the Pain Assessment Checklist for Seniors with Limited Ability to Communicate (PACSLAC)[68] and the DOLOPLUS-2 scales measuring pain behaviors in the elderly [69]. The above 2 widely used scales, when used together, collect a fair amount of useful data to understand the patient’s pain. The PACSLAC gathers information around facial expressions, activity/body movement, social (i.e. interpersonal relationships)/personality mood, physiological and/ or autonomic alterations including sleep, appetite etc.). Of note, the DOPOPLUS-2 importantly accounts for somatic, psychomotor, and psychosocial responses.
All the above elements are tested, in their own rights, to see if there are any major alterations which potentially may be the consequence of pain. Therefore, when used together, it appears these 2 instruments gather a fair amount of insight into the patient’s pain experiences, based on a biopsychosocial model. In 2011, the European Cooperation in Science and Technology’s (COST) action took up the task to develop a suitable toolkit to assess patients with impaired cognition, specifically patients with dementia. The main goal of this COST initiative was to create a comprehensive and internationally recognized assessment toolkit for the subtypes of dementia [11]. The final that was produced, the Pain assessment in Impaired Cognition (PAIC) tool attempts to assess a number of painrelated items under 3 categories: Facial expression, vocalization and body movements [70]. Unfortunately, this tool again appears tot to account for a full biopsychosocial evaluation of patients with AD and ODRDs. Corbett et al. [65] reviewed the first phase of the COST collaboration; a collection of the evidence from the literature and published pain assessment tools with consideration from clinical experts, to reach agreement, within Europe, on a core assessment instrument for pain in people with dementia (the PAIC) [65]. It was suggested that there were a number of potentially suitable tools, however there was no individual instrument that revealed the required reliability, validity or clinical appropriateness that was initially set out by the COST action panel [65]. The development of the initial PAIC did not begin with patient behavior observations but rather with dissection of existing validated peer reviewed pain assessment tools by well-thought-of experts in the field. Therefore, it was thought that there still was a level of validation data in a number of the tools that were investigated, and this provided the researchers with the capacity to develop a composite meta-tool; the PAIC-meta-tool [65].
The meta-tool was hence built on the premise of extracting the most pertinent sections of the existing validated tools [65]. The urgent need was to build on and advance on the prevailing evidence and available tools base rather than to establish a new tool from scratch thus, a meta-tool was created. Corbett et al. [65] add that the decision to create an in-depth tool kit to support the new meta-tool would be a vital aspect of this process. This would enable an assessment of other common symptoms of dementia which frequently overlap with pain-related symptoms. Specifically, Behavioral and Psychological Symptoms of Dementia (BPSD), such as agitation and aggression may be evaluated. In addition, the aim is that the toolkit will be tailored for use in patients with other neurodegenerative condition such as Parkinson’s Disease and Huntington’s Disease. Finally, the toolkit should also critically incorporate a section for assessment using self-report for those patients who are still cognitively able to provide information around their symptoms [65]. In addition, an important feature was to create a meta-tool with international agreement across many European countries. This is a crucial idea as a universal assessment instrument would allow for both standardization of clinical practice and assessment, neither of which were presently possible with the current pain assessment tools [65]. The researchers also suggest that the tool, at this point, will be translated into at least 7 European languages. However, with reference to the above, the meta-tool was developed according to ‘universal European’ standards which does not include cultural and language deviations in Non-European countries [65,71].
Therefore, validating this PAIC meta-tool in other non- European countries appears to be an important task moving forward along with the other important goals set out by the metatool, as described above. Therefore, a toolkit to assess individuals with AD and ODRDs that includes all the variables that Corbett et al. [65] describe should certainly be worked on further so to fully address the complexities underlying the assessment of either ‘communicative’ or ‘non-communicative’ patients’ pain who have AD and ODRDs Finally, Neuropsychiatric Symptoms (NPS) are a regular occurrence in many neurodegenerative diseases including dementia [72,73] and pain is thought to be a significant contributing factor [57,58]. Selbaek et al. found that close to all their study sample experienced clinically relevant NPS but individual symptoms differed. Furthermore, the researchers revealed that an increase in severity of dementia was correlated with an increase in severity in patients’ agitation, psychosis, and apathy. However, the exact relationship between pain and psychosis was still unclear and in addition the effect of pain treatment on symptoms of psychosis was also uncertain [56,58]. A further research study found that pain was a significant cause of psychosis, specifically delusions, in patients with dementia [74]. Secondly, it was revealed that pain appeared to be a major catalyst of agitation, including abnormal motor behaviors [74]. Finally, the administration of opioids to manage the pain appeared not to escalate the psychotic symptoms [74]. Therefore, when treating patients with AD and ODRDs who appear to also have neuropsychiatric symptoms associated with their primary conditions, it is vital to assess and understand how potentially pain, if present in the individuals, may be a major source of some of the maladaptive behaviors and thought processes in these types of patients. To identify the above, a clinician is required firstly to identify if there is pain in these individuals and if so, gain a full look into the patients’ pain perceptions. Therefore, once again, the need for a thorough biopsychosocial pain assessment process using instruments, tool kits and narrative interviews, to attain the full pain story for each individual.
Pain management Alzheimer’s disease and other dementia-related diseases
As noted, due to the scope of this paper, a condensed description around the topic of pain management for individuals with AD and ODRDs will now be executed. Generally, pain treatment in the older person is different to that of in the younger person [12]. As noted in a relatively new systematic review, there is a worrying state regarding pain management in specifically advanced cases of dementia [75]. With thorough pain evaluations being put in clinical practice in addition to observational pain tools very rarely being tested for reliability, validity and responsiveness, in particular, effective assessment of pain management strategies is therefore deficient [75]. Thus, there is a large gap in efficient appraisal of pain management modalities [75], including pharmacological and nonpharmacological interventions. Distinguishing between whether the perception of pain is either nociceptive (somatosensory or peripherally based pain), centrally based pain (spinal cord and/ or cortical origin) [1], or other including the neuroimmune system [44-46,76-79], for example, are critical to discern so to manage the biological sources of the pain. With reference to the evidence of the mechanisms underlying pain in patients with AD and ODRDs, the involvement of neurologists [1], and other necessary healthcare professionals to complete the much required interdisciplinary management model for such patients is vital. Furthermore, paying attention to the biopsychosocial model, examining the psychological, affective and social components that may be contributing to an individual’s pain experience, is key to unlocking and addressing an individual’s pain experience through a patientcentered model [80-82]. Due to the current limited attention given to pain experienced in these neurodegenerative diseases, there are very few, if any, reported randomized controlled trials around specific pain treatment guidelines and thus management of pain is generally based on symptoms (utilization of analgesics and anti-inflammatory drugs) and not structured around the correct pathophysiological mechanisms of the pain [1].
No disease-modifying agents have been demonstrated to be effective in AD [4]. It must be highlighted that pharmacokinetic and pharmacodynamic changes resulting from aging and a general decline in physiological function often make it difficult for this population to tolerate pain medication [5]. Husebo et al. [75], in their systematic review, found that expert groups predominantly support the use of various analgesic drugs such as paracetamol (acetaminophen), strong opioids and anticonvulsants [11,83-87] to treat pain in patients with dementia [75]. The outcome of their research revealed that despite the increase in analgesic usage in patients with AD and ODRDs, pain is still present in this group of individuals following such treatment. The systematic review still highlights the somewhat anemic evidence for the use of analgesics in dementia [75]. It must be noted that this is not an exhaustive list of medications that can be used with patients with pain who have AD and ODRDs. Others include Non-Steroidal Anti-Inflammatories (NSAIDs), corticosteroids and adjuvant analgesics (tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors and others), calcitonin, bisphosphonates, topical analgesics and benzodiazepines and more [5]. There appears to be a lack of well-constructed randomized controlled trials surrounding this topic and thus this does not allow for decisive clinical guidelines and protocols being implemented by clinical staff for this patient population [75]. Ultimately, there is varied evidence regarding all the drugs mentioned above, and thus care must be taken when prescribing these medications to patients with AD and ODRDs. The pharmacological management of pain in patients with dementia can be extremely involved due to comorbidities, polypharmacy, age-related pharmacodynamic and pharmacokinetic alterations that affect deciding upon what drug to use [5].
It is suggested that new pharmacological approaches able to increase pain thresholds and with decreased side effects may represent an effective substitute to treatment of CP in patients with AD and ODRDs [12]. Given the lack of clinical evidence that may be inferred based on clinical trials, the suggestions provided by the main current guidelines for pharmacological management, are still basically based on clinical experience [12]. With all this said, developing clinical guidelines based on rigid research should ultimately be based on the pain pathophysiological mechanisms at play in AD and ODRDs. Even more so, clinicians should be able to identify these mechanisms through a thorough biopsychosocial assessment of each individual patient in front of them, and in turn keep patient management within a patientcentered framework. Therefore, medication regimens should be individually-tailored with a regular evaluation of the regimen to assist in better pain control patients with AD and ODRDS [5]. Complexities of pain in older people with AD and ODRDs, requires an all-inclusive pain management approach that includes more than pharmacotherapy [14]. Furthermore, with recent concerns around the opioid pandemic with reference to the management of patients with CP, increased attention has been given to the use of nonpharmacological treatments [88]. With the limited efficacy of current pharmacological therapy and with the knowledge that caring for people with AD and ODRDs necessitates many healthcare professionals (psychologists, occupational therapists, physical therapists etc.), caregivers and immediate family, a comprehensive and individualized management is key [4]. Thus, research pertaining to non-pharmacological modalities has been [4] and continues to be held in high esteem. An interdisciplinary or multidisciplinary approach to tailored treatment for patients suffering from ADs and ODRDs, including managing symptoms of pain if present, is required to manage these patients. Institutions invested in an interdisciplinary team model to managing problems associated with AD and ODRDs, are best prepared to gain information that provides knowledge in order to navigate the treatment plan and in addition engages individuals most likely to be useful in executing the pain management proposal [14].
The inclusion of nondrug interventions entails careful deliberation of the specific patient circumstances, likings and evidence of efficacy and direction for selection of these modalities in the frail older person with AD and ODRDs [14]. A narrative review by Zuchella et al. [4], around the evidence for various nonpharmacological treatments for patients with AD and ODRDs, suggested that exercise and motor rehabilitation and most cognitive interventions showed the best, however moderate, outcomes [4]. Other treatments such as occupational therapy, psychological therapy, art therapy and others, all revealed small to very small improvements in patients’ symptoms [4]. Although there does appear to be a slow expansion of evidence around this topic, the majority of evidence for non-pharmacological interventions has been conducted using cognitively intact older adults because those with dementia are typically on the exclusion list in Randomized Controlled Trials (RCTs) [14]. Evidence is growing on non-pharmacological interventions to manage Behavioral And Psychological Symptoms Of Dementia (BPSD), but very few studies focus on pain as the primary outcome if interest [14]. The few studies that have been completed with a focus on pain in patients with AD and ODRDs, have found that exercise, behavioral-based treatments for BPDS such as music therapy, Cognitive Behavioral Therapy (CBT), reflexology, Reiki and others were effective in reducing pain and behavioral symptoms in patients with dementia [89-91]. A relatively recent review of RCTs that concentrated on complementary and alternative treatments to help manage pain and agitation in individuals with dementia, found that massage, touch and human interaction and physical presence, were useful in decreasing pain and agitation [92].
Although the evidence for the above treatment modalities is still limited in use with patients with AD and ODRDs, using strategies that the caregiver believes to be potentially valuable for the patient with AD and ODRDs, should be encouraged [14]. Importantly, psychological treatments has growing evidence in the older adult [93], however there is limited research when it comes to its use in patients with dementia [14]. Due to impairments in memory, language, executive function, visuospatial skills and other cognitive processes, engagement in psychological therapies such as CBT, mindfulness modalities and self-regulatory approaches (including biofeedback, relaxation exercises and hypnotherapy) are stunted [14]. Therefore it may be likely that these types of interventions may not be reasonable in patients with moderate to severe dementia [14], however there is certainly much room for further research in this area. With the onset of the COVID-19 pandemic and the further use of telemedicine, it seems that this use of technology may be an extremely reliable tool to aid in following up and treating patients with AD and ODRDs. Through using telehealth, patients and clinicians can be in contact far more frequently, therefore allowing for tighter management of these patients, via more frequent assessment of their symptoms either from the caregiver or actual patient themselves using digital diaries or personal digital assistants [18]. Novel telemedicine strategies have been found to be effective to navigate consultation and talk therapies and to instill rehabilitative pain self-management strategies [94-96]. Telehealth has been indicated to provide behavioral medicine interventions in CP patients through modalities that target both the sensory and affective elements of their pain experiences [97]. The above is of great relevance pertaining to the management of patients with AD and ODRDs through the biopsychosocial lens.
Conclusion
Despite its potential impact on the consequence of the disease and negative bearing on patients’ quality of life, pain is poorly studied in neurodegenerative diseases [1], including AD and ODRDs. Whether pain is acute or chronic, it may be hypothesized that pain can cause cognitive dysfunction in patients who have predementia or other communicative pathologies [32]. Pain in patients with AD and ODRDs may be catalyzed by a variety of entities including age-related musculoskeletal degeneration (although individuals can have early onset AD and ODRDs), immobility or neurodegeneration in cortical regions that are normally involved in analgesic responses [1], as discussed in this review. Pain certainly can reduce attention to the environment and is certainly associated with affective changes that can further limit cognitive ability [23,59- 63]. However, whether we entertain the ‘chicken or egg’ scenario, pain needs to always be respected and thus, assessed and managed through an interdisciplinary format. Achterberg et al. [11] state that in reference to patients with AD and dementia, it is “of critical importance to improve the recognition and assessment of pain to ensure that patients receive the most appropriate treatment”.
Interventions
DVD Intervention. The DVD arm of the intervention consisted of a three-disc set which included the thirteen edited classes previously filmed in the face-to-face Pain Education School program. The original, face-to-face Pain Education School was developed and implemented in a Department of Veterans Affairs (VA) Medical Center using the National Center for Health Promotion and Disease Prevention’s step-by-step guidelines in Veterans with chronic, non-cancer pain [17]. The face-to-face program consists of an introduction class followed by twelve hour-long classes offered weekly that are led by guest speakers from over twenty different disciplines within the facility. The face-to-face program lasts approximately three months’ time. Thirteen modules were created from the face-to-face intervention in order to provide the participant a menu of pain treatment options, including an introduction module followed by segments on Pain Clinic/Osteopathic Manipulation, Medication Management, Smoking Cessation/Addiction Services, Nutrition Services/MOVE! Weight Loss Program, Physical Medicine and Rehabilitation, Recreation Therapy/Sexual Health, Cognitive Behavioral Therapy (CBT)/Acceptance and Commitment Therapy Groups, Suicide Prevention and Mental Health/Vocational Rehabilitation, Hypnosis/Biofeedback, Healing Touch/Spirituality, Sleep Clinic/Insomnia CBT Group, and Acupuncture and Traditional Chinese Medicine. Each clip on the DVD from each discipline (30- 45 minutes each) shared information about chronic, non-cancer pain from their perspective, what treatments are available in their service, and how to access their respective clinics. Booklet Intervention. The booklet arm of the intervention only included the written materials (PowerPoint presentations) used with Veterans who participated in the same face-to-face intervention. The third arm of the intervention included both aspects aforementioned.
Although there are measures and instruments that are currently being used in this patient population, they do still have some pitfalls. One of the major challenges in recognizing, assessing and treating pain in patients with AD and ODRDs, is to develop an assessment toolkit that has strong psychometric properties, can be used with patients with differing types of cognitive and other comorbid problems, is available in many languages, is simple to use in different environments and, importantly, is practical for clinicians to use [11]. In turn, through establishing a valid and reliable battery of instruments to use when assessing patients with AD and ODRDs, specific consideration must be directed towards understanding the biopsychosocial pain mechanisms at play that contribute to an individualized patient-assessment model. Ultimately, a thorough examination of individuals with AD and ODRDs pain experiences, including predisposing factors and symptoms needs to be conducted using a biopsychosocial assessment model so as to fully appreciate a patient’s pain experience and therefore manage the symptoms in its entirety [98-102].
Through addressing all potential mechanisms of pain contributing to each patient’s personal pain experiences, a patientcentered treatment approach should be provided. This would importantly mean that correctly targeted drug administration as well as other non-pharmacological and non-invasive analgesic treatments are given only when necessary for the patient at hand. This would be based on the distinct findings through a thorough biopsychosocial pain assessment for each and every patient presenting with AD and ODRDs. In addition, if required, clinicians should acknowledge and advocate for their patients with neurodegenerative conditions such as AD and dementia to have their pain correctly treated. Furthermore, there is a significant need to instill support and transparent guidance for clinicians involved in treatment of patients with AD and ODRDs, in order to allow them to undertake informed decision making and minimize the reluctance to prescribe effective analgesics for their patients [11]. The above will only occur if pain mechanisms related to individual AD and ODRDs patients are fully understood by clinicians. Therefore, research pertaining to investigating the above is highly required and recommended.
References
- De Tommaso M, Arendt Nielsen L, Defrin R, Kunz M, Pickering G, et al. (2016) Pain in neurodegenerative disease: Current knowledge and future perspectives. Behav Neurol 2016: 27313396.
- Lautenbacher S (2014) Pain assessment in special patient groups such as those with dementia: At the finishing line or just starting from scratch? Pain 155(8): 1419-1420.
- Kristjanson LJ, Toye C, Dawson S (2003) New dimensions in palliative care: A palliative approach to neurodegenerative diseases and final illness in older people. Med J Aust 79(6): 41-43.
- Zucchella C, Sinforiani E, Tamburin S, Federico A, Mantovani E, et al. (2018) The multidisciplinary approach to alzheimer’s disease and dementia: A narrative review of non-pharmacological treatment. Front Neurol 9: 1058.
- Blair Sarbacker G (2014) Pain management in dementia. US Pharm 39(3): 39-43.
- Bunk S, Zuidema S, Koch K, Lautenbacher S, De Deyn PP, et al. (2021) Pain processing in older adults with dementia-related cognitive impairment is associated with frontal neurodegeneration. Neurobiol Aging 106: 139-152.
- Achterberg W, Lautenbacher S, Husebo B, Erdal A, Herr K (2021) Pain in dementia. Pain Rep 5(1): 803.
- Karantzoulis S, Galvin JE (2011) Distinguishing alzheimer’s disease from other major forms of dementia. Expert Rev Neurother 11(11): 1579-1591.
- Vinicius M, De Mello C, Vieira L, Cruz de Souza L, Gomes K, et al. (2019) Alzheimer’s disease: Risk factors and potentially protective measures. J Biomed Sci 26: 33.
- Ferreira D, Perestelo-Pérez L, Westman E, Wahlund LO, Sarrisa A, et al. (2014) Meta-review of CSF core biomarkers in Alzheimer’s disease: The state-of-the-art after the new revised diagnostic criteria. Front Aging Neurosci 6: 47.
- Achterberg P (2013) Clinical interventions in aging dove press pain management in patients with dementia. Clin Interv Aging 8: 1471-1482.
- Cravello L, Santo S, varrassi G, Benincasa D, Marchettini C, et al. (2019) Chronic pain in the elderly with cognitive decline: A narrative review. Pain Ther 8(1): 53-65.
- Van Kooten J (2016) A review of pain prevalence in Alzheimer’s, vascular, frontotemporal and Lewy body dementias. Dement Geriatr Cogn Disord 41(3-4): 220-232.
- Achterberg W, Lautenbacher S, Husebo B, Erdal A, Herr K (2020) Pain in dementia. Pain Reports 5(1): E803.
- Kress HG (2014) Managing chronic pain in elderly patients requires a CHANGE of approach. Curr Med Res Opin 30(6): 1153-1164.
- Westpha ECl, Alkema G, Seidel R, Chernof B, Goodwin C (2016) How to get better care with lower costs? See the person, not the patient. J Am Geriatr Soc 64(1): 19-21.
- IASP (2020) IASP announces revised definition of pain. International Association for the Study of Pain, USA.
- Scuteri D (2020) Pain assessment and treatment in dementia at the time of coronavirus disease covid-19. Front Neurol 11: 890.
- Cole LJ, Farrell MJ, Duff EP, Barber JB, Egan GF, et al. (2006) Pain sensitivity and fMRI pain-related brain activity in Alzheimer’s disease. Brain 129(11): 2957-2965.
- Kunz M, Mylius V, Scharmann S, Schelpelman K, Lautenbacher S (2009) Influence on dementia on multiple components of pain. Eur J Pain 13(3): 317-325.
- Ashar YK, Chang LJ, Wager TD (2017) Brain mechanisms of the placebo effect: An affective appraisal account. Annu Rev Clin Psychol 13: 73-98.
- Bolognini N, Olgiati E, Maravita A, Ferraro F, Fregni F (2013) Motor and parietal cortex stimulation for phantom limb pain and sensations. Pain 154(8): 1274-1280.
- Kucyi A, Moayedi M, Fogel LW, Goldberg MG, Freeman BV, et al. (2014) Enhanced medial prefrontal-default mode network functional connectivity in chronic pain and its association with pain rumination. J Neurosci 34(11): 3969-3975.
- Roussel N, Nijs J, Meeus M, Mylius V, Fayt C, Oostendorp R (2013) Central sensitization and altered central pain processing in chronic low back pain: Fact or myth? Clin J Pain 29(7): 625-638.
- Yoshino A (2018) Changes in resting-state brain networks after cognitive-behavioral therapy for chronic pain. Psychol Med 48(7): 1148-1156.
- DeCharms C (2005) Control over brain activation and pain learned by using real-time functional MRI. PNAS 102(51): 18626-18631.
- Nees F, Usai K, Löffler M, Flor H (2019) The evaluation and brain representation of pleasant touch in chronic and subacute back pain. Neurobiol Pain 5: 100025.
- Steeds CE (2016) The anatomy and physiology of pain. Surg 34(2): 55-59.
- De Ridder D, Adhia D, Vanneste S (2021) The anatomy of pain and suffering in the brain and its clinical implications. Neurosci Biobehav Rev 130: 125-146.
- Yang S, Chang MC (2019) Chronic pain: Structural and functional changes in brain structures and associated negative affective states. Int J Mol Sci 20(13): 3130.
- Kunz M, Mylius V, Schepelmann K, Lautenbacher S (2015) Loss in executive functioning best explains changes in pain responsiveness in patients with dementia-related cognitive decline. Behav Neurol 2015: 878157.
- González LCA (2011) The neurologist facing pain in dementia. Neurol 30(9): 574-585.
- Treede RD, Apkarian AV, Bromm B, Greenspan JD, Lenz FA (2000) Cortical representation of pain: Functional characterization of nociceptive areas near the lateral sulcus. Pain 87(2): 113-119.
- Peyron R, Laurent B, García-Larrea L (2000) Functional imaging of brain responses to pain. A review and meta-analysis. Neurophysiol Clin 30(5): 263-288.
- Scherder EJA, Sergeant JA, Swaab DF (2003) Pain processing in dementia and its relation to neuropathology. Lancet Neurol 2(11): 677-686.
- Monroe T, Gore J, Chen LM, Milon L, Cowan R (2012) Pain in people with alzheimer disease: Potential applications for psychophysical and neurophysiological research Todd. J Geriatr Psychiatry Neurol 25(4): 240-255.
- Parvizi J, Van Hoesen GW, Damasio A (2000) Selective pathological changes of the periaqueductal gray matter in Alzheimer’s disease. Ann Neurol 48(3): 344-353.
- Ishunina TA, Swaab DF (2002) Neurohypophyseal peptides in aging and Alzheimer’s disease. Ageing Res Rev 1(3): 537-558.
- Krueger CE (2010) Longitudinal rates of lobar atrophy in frontotemporal dementia, semantic dementia and alzheimer’s disease. Alzheimer Dis Assoc Disord 24(1): 43-48.
- Scherder E (2005) Recent developments in pain in dementia. Br Med J 330(7489): 461-464.
- Lawn T (2021) Pain in the neurodegenerating brain: Insights into pharmacotherapy for Alzheimer disease and Parkinson disease. Pain 162(4): 999-1006.
- Mitchell AJ, Monge-Argilés JA, Sánchez-Paya J (2010) Do CSF biomarkers help clinicians predict the progression of mild cognitive impairment to dementia? Pract Neurol 10(4): 202-207.
- McKhann G (2012) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging- Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7(3): 263-269.
- Gao YJ, Ji RR (2010) Targeting astrocyte signalling for chronic pain. Neurotherapeutics 7(4): 482-493.
- Scholz J, Woolf CJ (2007) The neuropathic pain triad: Neurons, immune cells and glia. Nat Neurosci 10(11): 1361-1368.
- Wei F, Guo W, Zou S, Ren K, Dubner R (2008) Supraspinal glial-neuronal interactions contribute to descending pain facilitation. J Neurosci 28(42): 10482-10495.
- Totsch SK, Sorge RE (2017) Immune system involvement in specific pain conditions. Mol Pain 13: 1744806917724559.
- Graeber MB, Christie MJ (2012) Multiple mechanisms of microglia: A gatekeeper’s contribution to pain states. Exp Neurol 234(2): 255-261.
- Cao S, Fisher DW, Yu T, Dong H (2019) The link between chronic pain and Alzheimer’s disease. J Neuroinflammation 16(1): 204.
- Millan MJ (2001) Descending control of pain. Prog Neurobiol 66(6): 355-474.
- Kirkpatrick DR (2015) Therapeutic basis of clinical pain modulation. Clin Transl Sci 8(6): 848-856.
- Ossipov M, Dussor G, Porreca F (2010) Central modulation of pain. J Clin Invest 120(11): 3779-3787.
- Ossipov M, Morimura K, Porreca F (2014) Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care 8(2): 143-151.
- Kwon M, Altin M, Duenas H, Alev L (2014) The role of descending inhibitory pathways on chronic pain modulation and clinical implications. Pain Pract 14(7): 656-667.
- Pertovaaraa A, Almeida DA (2006) Endogenous pain modulation: Descending inhibitory systems. Handb Clin Neurol 81(3): 179-192.
- Kinney M, Seider J, Beaty AF, Coughlin K, Dyal M, et al. (2020) The impact of therapeutic alliance in physical therapy for chronic musculoskeletal pain: A systematic review of the literature. Physiother Theory Pract 36(8): 886-898.
- Ardito RB, Rabellino D (2011) Therapeutic alliance and outcome of psychotherapy: Historical excursus, measurements, and prospects for research. Front Psychol 2: 270.
- De Bolle M, Johnson JG, De Fruyt F (2010) Patient and clinician perceptions of therapeutic alliance as predictors of improvement in depression. Psychother Psychosom 79(6): 378-385.
- Howgego IM, Yellowlees P, Owen C, Meldrum L, Dark F (2003) The therapeutic alliance: The key to effective patient outcome? A descriptive review of the evidence in community mental health case management. Aust N Z J Psychiatry 37(2): 169-183.
- Del Re AC, Flückiger C, Horvath AO, Symonds D, Wampold BE (2012) Therapist effects in the therapeutic alliance-outcome relationship: A restricted-maximum likelihood meta-analysis. Clin Psychol Rev 32(7): 642-649.
- Diener I, Kargela M, Louw A (2016) Listening is therapy: Patient interviewing from a pain science perspective. Physiother Theory Pract 32(5): 356-367.
- Eveleigh RM, Muskens E, Van Ravesteijn H, Van Dijk I, Van Rijswijk E, et al. (2012) An overview of 19 instruments assessing the doctor-patient relationship: Different models or concepts are used. J Clin Epidemiol 65(1): 10-15.
- Room MJ (2023) The importance of harnessing neurophysiological placebo analgesic mechanisms through therapeutic contextual factors when treating patients with chronic pain, 8(1): 20-25.
- Jensen Dahm C (2014) Quantitative sensory testing and pain tolerance in patients with mild to moderate Alzheimer disease compared to healthy control subjects. Pain 155(8): 1439-1445.
- Corbett A (2014) An international road map to improve pain assessment in people with impaired cognition: The development of the Pain Assessment in Impaired Cognition (PAIC) meta-tool. BMC Neurol 14(1): 229.
- Hadji Stavropoulos T (2007) An interdisciplinary expert consensus statement on assessment of pain in older persons. Clin J Pain 23(1): 1-43.
- Pergolizzi J (2008) Opioids and the management of chronic severe pain in the elderly: consensus statement of an international expert panel with focus on the six clinically most often used world health organization step iii opioids (buprenorphine, fentanyl, hydromorphone, met. Pain Pract 8(4): 287-313.
- Fuchs-Lacelle S, Hadjistavropoulos T (2004) Development and preliminary validation of the Pain Assessment Checklist for Seniors with Limited Ability to Communicate (PACSLAC). Pain Manag Nurs 5(1): 37-49.
- Wary B, Doloplus C (1999) Doloplus-2, a scale for pain measurement. Soins Gerontol 19: 25-27.
- Kappesser J, Voit S, Lautenbacher S, Hermann C (2020) Pain assessment for cognitively impaired older adults: Do items of available observer tools reflect pain-specific responses? Eur J Pain 24(4): 851-862.
- Van Dalen-kok AH (2018) Pain Assessment in Impaired Cognition (PAIC): Content validity of the Dutch version of a new and universal tool to measure pain in dementia. Clin Interv Aging 13: 25-34.
- Selbæk G, Engedal K, Benth JS, Bergh S (2014) The course of neuropsychiatric symptoms in nursing-home patients with dementia over a 53-month follow-up period. Int Psychogeriatrics 26(1): 81-91.
- Vos T (2016) Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: A systematic analysis for the global burden of disease study 2015. Lancet 388(10053): 1545-1602.
- Habiger TF, Flo E, Achterberg WP, Husebo BS (2016) The interactive relationship between pain, psychosis and agitation in people with dementia: Results from a cluster-randomised clinical trial. Behav Neurol 2016: 7036415.
- Husebo BS, Achterberg W, Flo E (2016) Identifying and managing pain in people with alzheimer’s disease and other types of dementia: A systematic review. CNS Drugs 30(6): 481-497.
- Hanani M, Spray DC (2020) Emerging importance of satellite glia in nervous system function and dysfunction. Nat Rev Neurosci 21(9): 485-498.
- Ji NM, Berta R, Nedergaard M (2013) Glia and pain: Is chronic pain a gliopathy? Pain 154(1): S10-S28.
- Laumet G, Ma J, Robison AJ, Kumari S, Heijnen CJ, et al. (2019) T cells as an emerging target for chronic pain therapy. Front Mol Neurosci 12: 216.
- Hore Z, Denk F (2019) Neuroimmune interactions in chronic pain-An interdisciplinary perspective. Brain Behav Immun 79: 56-62.
- Lumley M (2011) Pain and emotion: A biopsychosocial review of recent research. J Clin Psychol 67(9): 942-968.
- Dorflinger L, Kerns RD, Auerbach SM (2013) Providers’ roles in enhancing patients’ adherence to pain self-management. Transl Behav Med 3(1): 39-46.
- Butera KA, Roff SR, Buford TW, Cruz Almeida Y (2019) The impact of multisite pain on functional outcomes in older adults: Biopsychosocial considerations. J Pain Res 12: 1115-1125.
- Herr K, Coyne PJ, McCaffery M, Manworren R, Merkel S (2011) Pain assessment in the patient unable to self-report: Position statement with clinical practice recommendations. Pain Manag Nurs 12(4): 230-250.
- Herr K, Bjoro K, Decker S (2006) Tools for assessment of pain in nonverbal older adults with dementia: A state-of-the-science review. J Pain Symptom Manage 31(2): 170-192.
- AGS Panel in persistent Pain in Older Persons (2002) The management of persistent pain in older persons. J Am Geriatr Soc 50(6): 205-224.
- American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons (2009) Pharmacological management of persistent pain in older persons. J Am Geriatr Soc 57(8): 1331-1346.
- Abdulla A, Bone M, Adams N, Elliott AM, Jones D, et al. (2013) Evidence-based clinical practice guidelines on management of pain in older people. Age Ageing 42(2): 151-153.
- Tick H (2018) Evidence-based nonpharmacologic strategies for comprehensive pain care: The consortium pain task force white paper. Explore 14(3): 177-211.
- Dyer SM, Harrison SL, Laver K, Whitehead C, Crotty M (2018) An overview of systematic reviews of pharmacological and non-pharmacological interventions for the treatment of behavioural and psychological symptoms of dementia. Int Psychogeriatrics 30(3): 295-309.
- Legere LE, McNeill S, Schindel Martin L, Acorn M, An D (2018) Nonpharmacological approaches for behavioural and psychological symptoms of dementia in older adults: A systematic review of reviews. J Clin Nurs 27(7-8): e1360-e1376.
- Pieper MJC, Dalen Kok AH, Francke AL, Steen JT, Scherder EJA, et al. (2013) Interventions targeting pain or behaviour in dementia: A systematic review. Ageing Res Rev 12(4): 1042-1055.
- Anderson AR, Deng J, Anthony RS, Atalla SA, Monroe TB (2017) Using complementary and alternative medicine to treat pain and agitation in dementia: A review of randomized controlled trials from long-term care with potential use in critical care. Crit Care Nurs Clin North Am 29(4): 519-537.
- Bieu RK, Kulas JF (2019) Handbook on the neuropsychology of aging and dementia. (2nd edn), Gewerbesrasse: Springerlink, p. 779.
- Gardner J, Backman S, Barbati J, Grummitt J (2008) Evaluating distance education of a mindfulness-based meditation programme for chronic pain management. J Telemed Telecare 14(2): 88-92.
- Mcgeary DD, Mcgeary CA, Gatchel RJ (2012) A comprehensive review of telehealth for pain management: Where we are and the way ahead. Pain Pract 12(7): 570-577.
- Rosser BA, McCullagh P, Davies R, Mountain GA, McCracken L, et al. (2011) Technology-mediated therapy for chronic pain management: The challenges of adapting behaviour change interventions for delivery with pervasive communication technology. Telemed e-Health 17(3): 211-216.
- Appel PR, Bleiberg J, Noiseux J (2002) Self-regulation training for chronic pain: Can it be done effectively by telemedicine? Telemed J e-Health 8(4): 361-368.
- Mills SEE, Nicolson KP, Smith BH (2019) Chronic pain: A review of its epidemiology and associated factors in population-based studies. Br J Anaesth 123(2): e273-e283.
- Crofford LJ (126) Chronic pain: Where the body meets the brain. Trans Am Clin Climatol Assoc 126: 167-183.
- Williams ACDC, Fisher E, Hearn L, Eccleston C (2020) Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev 11(11): CD007407.
- Larsson B, Dragioti E, Gerdle B, Björk J (2019) Positive psychological well-being predicts lower severe pain in the general population: A 2-year follow-up study of the SwePain cohort. Ann Gen Psychiatry 18(1): 8.
- Eccleston C, Hearn L, Williams ACDC (2014) Psychological therapies for the management of chronic neuropathic pain in adults. Cochrane Database Syst Rev 2015(10): CD011259.
© 2023 Marnin Joseph Romm. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






