- Submissions

Full Text
Developments in Anaesthetics & Pain Management
Appraisal of Nanotechnology Application to Local Anesthesia (LA)
Mohamed Sikkander A1*, Bassyouni F2 and Abdel El Rehim M3
1Department of Chemistry, Velammal Engineering College, India
2Department of Chemistry of Natural and Microbial Products,Pharmaceutical Research and drug Industries Institute,National Research Centre, Egypt
3Department of Materials and Nanophysics, KTH Royal Institute of Technology, Sweden
*Corresponding author: Mohamed Sikkander A, Department of Chemistry, Velammal Engineering College, India
Submission:January 25, 2023;Published: February 09, 2023

ISSN: 2640-9399 Volume2 Issue3
Abstract
Numerous improvements have been made to the formulation of local anaesthetics, and practical use has been made of their effectiveness in producing long-lasting motor and sensory block. By providing analgesia for a longer period of time with a single dosage, sustained release formulations help to avoid the problems that frequently occur when using conventional analgesics. Additionally, it is claimed that controlled release of an anaesthetic agent lessens side effects, particularly cardiotoxicity, neurotoxicity, and tissue lesions, and prevents overdosing. The use of liposomal formulation and nanotechnology has produced highly effective pain management and rapid patient recovery.
Introduction
Due to its importance for both physical and mental health, pain has been referred to as “the fifth vital sign” since 1996. Multimodal analgesia is a notion that has been introduced to address the opioid epidemic that is occurring during traditional pain management [1]. One of the most popular and secure analgesics in multimodal regimens are Local Anaesthetics (LAs). However, its application is constrained by its short half-life (less than 24 hours) and potential toxicity (cardiac and neurological system dysfunction), which increase the urgency of finding ways to balance the negative effects and sustained analgesia [2]. Although disposable catheters with pumps are used to extend the time between LAs, there is still a chance of catheter dislodgment, infection, and trauma [3]. Catheter placement is also labor and time intensive. The above-mentioned are offset by extended-release and its mechanism of LAs (Table1); (Figure1). In order to provide low systemic toxicity, they are capable of constantly releasing a safe amount with a single administration (often injection without general anaesthesia [4]. Long-lasting nociceptive block can be obtained in the meanwhile [5]. Peripheral nerves are the first stops to perceive pain stimuli during pain transmission. It is reasonable to consider inhibiting pain from the very beginning, stopping downstream reactions and maladaptive changes of neuroplasticity which are more difficult to control [6]. Therefore, LAs become a perfect choice. LAs work on peripheral nerves via binding to intracellular domain of voltagegated Na channel, inhibiting influx of Na+, resulting in the blockade of depolarization. LAs are constituted with three chemical groups: a hydrophilic amino group (mostly tertiary amines), a lipophilic benzene ring, and a linker which can be an amide or an ester, determining LAs’ classification [7].
Table 1: Advances of nano-structured extended-release local anesthetics.
Amide-type LAs are the most commonly used, including bupivacaine, ropivacaine, lidocaine, and mepivacaine. Estertype LAs involve chloroprocain, procaine, and tetracaine [8]. The earliest points of transmission for pain sensations are peripheral nerves. It makes sense to think about blocking pain right away in order to prevent downstream effects and more difficult-to-control maladaptive alterations in neuroplasticity [9]. LAs are a wonderful choice as a result. By attaching to the intracellular region of the voltage-gated Na channel and blocking the influx of Na+, LAs limit depolarization in peripheral neurons [10]. Three chemical groups make up LAs: a lipophilic benzene ring, a hydrophilic amino group (most commonly tertiary amines), and a linker that can either be an amide or an ester. The most popular LAs are those of the amide type, such as bupivacaine, ropivacaine, lidocaine, and mepivacaine [11]. Chloroprocain, procaine, and tetracaine are components of ester-type Las (Figure 1).
Figure 1:Extended-release and its mechanism of Las.
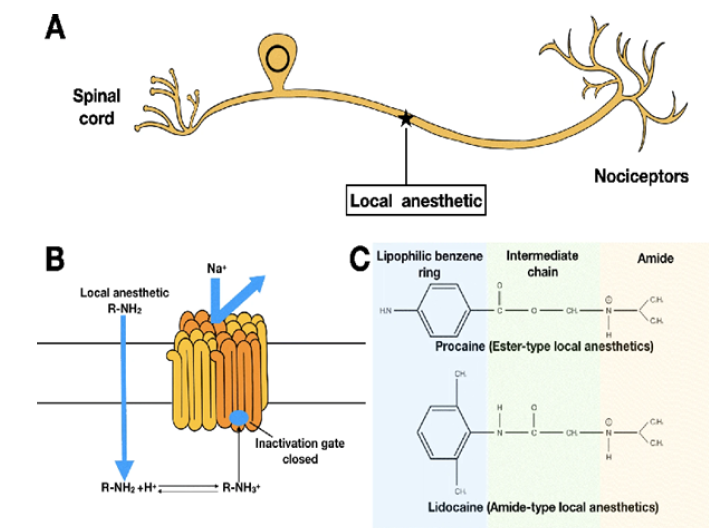
LAs have a significant role in multimodal analgesia. Its utilisation is constrained by a short duration and unfavorable side effects, which leads to the development of extended-release LAs [12]. Due to their similar size to the physiological environment, nano-structured DDSs exhibit greater biocompatibility and biodegradation compared to micro-structured DDSs [13]. Liposomes, a class of nanocarriers, have the first success in producing super-long-lasting LAs that can release bupivacaine for up to 72 hours invivo. Additionally, liposomes enhance the safety of LAs under emulsion protection [14]. However, liposome’s instability makes it difficult to store and co-administer with additional free LAs. Polymersomes have a more favourable profile than liposomes, with higher stability and longer release. Additionally, the electrospinning process and the stimuliresponsive characteristic give polymersomes greater flexibility in their form and release behavior [15]. Combining nanocarriers is an alternate method of improving flaws and enhancing strengths to materials and industrial processes. The stage is now set for hybrid nanocarriers, which not only enhance the release profile but also expand the range of administration methods, such as the transdermal route [16]. Future extended-release formulations for varied analgesic needs may be more precise and regulated thanks to the ever-growing versatility of nanocarriers.
References
- Owen GT, Bruel BM, Schade CM, Eckmann SM, Hustak EC, et al. (2018) Evidence-based pain medicine for primary care physicians. Proc (Bayl Univ Med Cent) 31(1): 37-47.
- Boghdadly K, Pawa A, Chin KJ (2018) Local anesthetic systemic toxicity: current perspectives. Local Reg Anesth 11: 35-44.
- Hazmi H (2015) Role of duration of catheterization and length of hospital stay on the rate of catheter-related hospital-acquired urinary tract infections. Res Rep Urol 7: 41-47.
- Macfarlane AJR, Gitman M, Bornstein KJ, Boghdadly K, Weinberg G (2021) Updates in our understanding of local anaesthetic systemic toxicity: A narrative review. Anesthesia 76(1): 27-39.
- Pérez BA, Deulofeu M, Homs J, Merlos M (2022) Long-lasting reflexive and nonreflexive pain responses in two mouse models of fibromyalgia-like condition. Sci Rep 12(1): 9719.
- Fenton BW, Shih E, Zolton J (2015) The neurobiology of pain perception in normal and persistent pain. Pain Management 5(4): 297-317.
- Körner J, Albani S, Eswaran VSB, Roehl AB, Rossetti G, et al. (2022) Sodium channels and local anesthetics-old friends with new perspectives. Front Pharmacol 13: 837088.
- Pete DD, Souza MS (2020) Local anesthetics. Side Effects of Drugs Annual 42: 155-163.
- Finnerup NB, Kuner R, Jensen TS (2021) Neuropathic pain: From mechanisms to treatment. Physiological Reviews 101(1): 259-301.
- https://www.nysora.com/topics/pharmacology/clinical-pharmacology-local-anesthetics/
- https://emedicine.medscape.com/article/873879-overview
- Kaye AD, Urman RD, Rappaport Y, Siddaiah H, Cornett EM, et al. (2019) Multimodal analgesia as an essential part of enhanced recovery protocols in ambulatory settings. J Anaesthesiol Clin Pharmacol. 35(1): S40-S45.
- Patra JK, Das G, Fraceto LF, Campos EVR, Torres MPR, et al. (2018) Nano based drug delivery systems: Recent developments and future prospects. J Nanobiotechnology 16: 71.
- Liu P, Chen G, Zhang J (2022) A review of liposomes as a drug delivery system: Current status of approved products, regulatory environments, and future perspectives. Molecules 27(4): 1372.
- Aibani N, Khan TN, Callan B (2020) Liposome mimicking polymersomes: A comparative study of the merits of polymersomes in terms of formulation and stability. Int J Pharm X 2: 100040.
- Fei Qu, Rui Geng, Yijing Liu, Jintao Zhu (2022) Advanced nanocarrier and microneedle-based transdermal drug delivery strategies for skin diseases treatment. Theranostics 12(7): 3372-3406.
© 2023 Mohamed Sikkander A. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






