- Submissions

Full Text
Developments in Anaesthetics & Pain Management
SARS-CoV-2 Pathobiology and Covid-19 Chemotherapy in Animals
Feliks Kania B* and Bacławska I
Veterinary Institute, University Center of Veterinary Medicine, Poland
*Corresponding author: Feliks Kania B, Veterinary Institute, University Center of Veterinary Medicine, Poland
Submission: July 12, 2021;Published: August 02, 2021

ISSN: 2640-9399 Volume2 Issue1
Abstract
SARS-CoV-2, which was identified in 2019 is responsible for the ongoing global pandemic. The zoonotic potential of Covid-19 raised many concerns on what was the origin of the situation we are currently living in, how can humans prevent it on a global scale and how to protect animals from spreading it further and causing new, potentially fatal mutations. It is crucial to discuss pathobiology, treatment as well as zoonotic aspects of this virus which is spreading worldwide at a threatening speed.
Keywords: Coronaviruses; Covid-19; SARS-CoV-2; Zoonosis; Immunology
What is SARS-COV-2 and COVID-19?
SARS-CoV-2 is the third large zoonotic Coronavirus (CoV) that has spread around the world within several weeks, causing a global pandemic at the end of 2019. Publicly available scientific sources indicate that SARS-CoV-2 has its origins in Wuhan, China. The first cases of an as yet unidentified pathogen began on November 17, 2019. The virus spread via zoonotic transmission between animals and humans. The first clinical case was an infected 55-year-old man who was a resident of Wuhan, in the Chinese province of Hubei. Subsequently, several further cases of similar diseases were reported in the same region, they were characterized by similar clinical symptoms of pneumonia of unknown etiology. The Chinese authorities linked the cases to a fish market in the area described above - a place where seafood (Frutti di mare) is sold and live animals traded. An epidemiological investigation initiated by the China Center for Disease Control and Prevention showed on January 7, 2020, that the problem is related to a newly isolated virus from the coronavirus group, working under the working name 2019-nCoV. A few days later, scientists already had a fully sequenced genome from sick patients. In the epithelium of the respiratory tract of patients, a genome very similar to B-line -coronaviruses, previously isolated from bats, was found-the SARS-like CoV bat. The virus shows high variability, which makes it highly susceptible to transmission between different species [1,2]. Covid-19 is classified as an acute infectious respiratory disease caused by the SARS-CoV-2 virus described above. The name Covid-19 was announced by the World Health Organization (WHO) and is now widely, officially used. “What” in the name means the corona, “vi”- virus, “d” - disease. The number 19 indicates the year of the appearance of the virus - 2019 (Corona-Virus-Disease-2019).
Molecular Structure of SARS-COV-2
The SARS-CoV-2 virus in its structure has an envelope and single-stranded RNA of positive
polarity. The virion shape is pleomorphic, in diameter 6-140nm. It is surrounded by spikes
9-12nm long. The genome length ranges from 29,867 to 29,903 nucleotides. It is with RNA of
viruses on one of these and tested. This genome encodes four structural, non-structural and
accessory proteins:
1. S (spike)-fusion protein or surface glycoprotein-responsible for interaction with the
receptor on the surface of cells,
2. E (envelope)-coat protein-responsible e.g., for forming virions,
3. M (membrane)-membrane proteins or membrane-the main matrix protein of the
virus,
4. N (nucleocapsid)-protein of nucleocapside-protecting
a large RNA molecule and participating in the modification of
cellular processes and viral replication.
The N protein holds the RNA genome, and the S, E, and M proteins
together form the viral envelope. The S protein is responsible for
binding to the host cell’s membrane [3].
SARS-COV-2 Transmission
SARS-CoV-2 was originally derived from bats. An attempt is proven to be a recipe for bat coronavirus. Due to their origin, nonanimal CoVs can be transmitted from animal to animal (veterinary infectious diseases), or to humans (zoonotic) and to humans (anthropozoones).
Animal to animal transmission
The current place in the world that coronaviruses will circulate in the world, the origin of CoV remains unclear represents several theories about the origin of SARS-CoV-2 disease. Originally thought to be come from bats, seafood, and snakes that have spread among people visiting or living in Wuhan, China, it has transmitted from pangolins to humans.
Animal to human transmission
The main mode of transmission of HCoV is the direct contact the animal with the host of the animal, animal contact or urine, consumption of milk or unprocessed meat from animals infected. SARS-CoV-2 was transmitted directly to humans by dromedaries, camels and pangolins, respectively [4,5].
Human to human transmission
SARS-CoV-2 is transmitted by human contact or by touching the contaminated surfaces by an infected person and by aerosol transmission. Transmission by direct contact with human is the turn of the caregivers of the healthcare workers and primary caregivers of the sick patient. This virus meets by droplets when coughing or coughing. Human transit can also follow contamination from polluted environments. The transfer of the spray to the next, when a sick person sneezes or coughs, is formed in ten droplets of the virus, which is thrown in the air at the top up to 1 meter and gets on the mechanism of finding the nose, eyes and learning to participate in nearby people.
Human-animal transmission
While SARS-CoV-2 is said to be transmitted to humans from cave-dwelling bat species, recent reports have raised concerns about reverse zoonotic transmission to fur animals, zoo animals, and pets such as dogs and cats. Continuation of research is needed to significantly prove this hypothesis worldwide by appropriately holistic approach to different possibilities and probabilities under different environmental conditions.
Pathobiology of Sars-Cov-2 Infection
SARS-CoV-2 binding with ACE2 receptors to perform host cells. The ACE2 and S protein binding receptor in SARS-CoV-2 is very similar to SARS-CoV. This shows that they share the same penetration mechanism into the host cells. The association of SARSCoV- 2 S with ACE2 has about 10 times the binding capacity due to SARS-CoV. It may also be one of the other factors that may favor multiple rates transmission of SARS-CoV-2 than SARS-CoV [6]. In the respiratory tract, in the case of SARS-Cov-2, ACE2 is widely expressed in the tracheal epithelium, bronchus, alveoli, alveolar monocytes, and bronchial serous glands and macrophages [7]. The SARS-CoV-2 virus replicates in these target cells, and then mature virions are released from primary cells and infect new target cells. In the case of SARS-CoV-2 respiratory secretions, droplets formed when sneezing and/or coughing, the stool, sweat and urine infected patients are infectious primarily to children of their own. Infectious droplets can be taken to a distance of ~1m or more due to air currents and can infect nearby surfaces and objects, so there is a risk of transmission of infection.
With regard to SARS-CoV-2 infection, the clinical pathology can be divided into three stages, namely: mild, severe and critical conditions. In a mild stage of infection, a patient may develop pneumonia, sometimes with symptoms of upper respiratory tract infection, characterized by a mild illness with fever, dry cough, sore throat, etc. In these cases, the respiratory rate will be greater than 30min due to shortness of breath , productive cough and hypoxia. These symptoms develop within a short time (24-48 hours). Finally, a critical stage involving severe pneumonia, respiratory failure, cardiac arrest and/or multiorgan failure leading to death.
Endothelial infection
In cases of Covid-19, the presence of virus particles in the endothelium and histological evidence of superficial and deep perivascular lymphocytic infiltrates and damage to the dermis with exocytosis are observed. Covid-19 infection leads in many cases to endothelial dysfunction. It acts as a direct target of the virus and inflammatory cytokines. It also results in the creation of a proinflammatory and procoagulant state in patients with Covid-19.
Pathologies-heart, brain, liver
In some infected patients, microvascular steatosis and mild lobular and portal activity have been reported in the liver tissues, interstitial mononuclear inflammatory infiltrates have been found in the heart tissues. Endothelial cell involvement has also been reported in various organs, including the kidneys, lungs, heart and liver.
Disseminated intravascular coagulation
A hemostatic system has been identified as contributing to severe Covid-19 pneumonia. The manifestation of Disseminated Intravascular Coagulation (DIC) appears to be responsible for the exacerbation of Covid-19 pneumonia and is often fatal. It has been reported that the coagulation system is activated in many Covid-19 patients, leading to higher D-dimer levels in patient non-survivors than in survivors. In some case reports of clinical cases, thrombosis and pulmonary embolism have been reported. Hemostatic abnormalities associated with Covid-19 include mild thrombocytopenia and elevated levels of D-dimer, both of which are associated with a higher risk of mechanical ventilation, intensive care, or death. Therefore, thromboprophylaxis is considered an appropriate option in the management of patients with Covid-19. According to laboratory findings, >70% of critical Covid-19 cases who became infected had disseminated intravascular coagulation. Another multicenter study conducted in the USA also showed that critically ill patients with Covid-19 suffered from thrombosis and bleeding [8,9].
Septic syndrome
Vascular endothelial cells play a central role in several physiological processes. They control blood rheology, regulation of vasomotor tone, osmotic balance and vascular barrier function. Likewise, endothelial cells play a key role in innate immune responses such as sepsis, as well as in activating acquired immunity. Endothelial cells are an important target of infection for most viruses diagnosed in humans, including SARS-CoV-2. By enhancing the immune response, they induce increased tissue permeability, inflammation and contribute to the severity of the viral disease. Covid-19 infection causes a variety of clinical symptoms. In many infected cases, it causes asymptomatic or mild symptoms. In a few cases, immune complications such as macrophage activation syndrome, also known as secondary haemophagocytic lymphohistiocytosis, result in cytokine storm syndrome and ARDS, which are fatal if not treated effectively.
SARS- COV-2 in Animals
In the animal world - as it turned out - there are 26 species which, having regular contact with infected people, may be susceptible to infection. Angiotensin 2 converting enzyme has been identified as the SARS-CoV-2 receptor. Binding to this protein allows the virus to enter host cells. Researchers looked at how the protein that spikes SARS-CoV-2 interacts with the ACE2 protein, which it attaches to when it infects humans. Scientists focused on whether mutations in the ACE2 protein of different animals that make it different from the human version reduce the stability of the viral protein-host complex. In the published results, the authors showed that the following species are susceptible to infection: golden snub-nosed monkey, king macaque (rhesus macaque), ermine (stoat), Chinese paguma (masked palm civet), rhinolophus macrotis (bigeared horseshoe bat), rhinolophus sinicus (Chinese rufous horseshoe bat), rousettus leschenaultii (Leschenault’s rousette), Eurasian wild boar, domestic pig, ferret, domestic dog, domestic cat (cat), Javanese pangolin, rhinolophus pearsonii (Pearson’s horseshoe bat), large flying fox, Sumatran orangutan, horse, domestic cattle, common chimpanzee , sheep, olive baboon, rabbit, red fox, Campbell’s hamster, golden hamster, white-eared uistiti (common marmoset), sand shark ( naked mole-rat), sandstone leopards (thirteen-lined ground squirrel) and Chinese hamster.
SARS-CoV-2 in dogs
On February 28, 2020, the Pomeranian dog tested positive for SARS-CoV-2 in Hong Kong. The animal showed no signs of disease. The genetic sequences of SARS-CoV-2 obtained from both the dog and its owners were very similar, indicating human-toanimal transmission of the Covid-19 virus. It was the first such report. Nasal and oral samples were “weakly positive” for SARSCoV- 2 in RT-PCR tests. Hence, the possibility of transmitting such a mild form of the disease to animals or humans, especially in the absence of any significant clinical symptoms, is very low. SARSCoV- 2 has also been detected in a Hong Kong German Shepherd Dog. In both of these cases, positive dogs lived in close contact with their positive Covid-19 owners. In 2020, Goumenou et al. [10] hypothesized that dogs could act as intermediate hosts in the transmission of SARS-CoV -2 in the human population in Italy. This hypothesis was based on the finding that the exponential increase in positives and deaths continued in Italy even with strict travel restrictions. This hypothesis was further supported by facts such as the close similarity of ACE2 in humans and dogs. In Italy, there is one dog for every six individuals, and the presence of human-toanimal transmission suggests the possibility of animal-to-human transmission.
SARS-COV-2 in Cats
SARS-CoV-2 was detected in two cats, one from Belgium and one from Hong Kong [11,12]. Scientists at the Harbin Veterinary Research Institute reported that cats can become infected with SARS-CoV-2 and transfer it to other cats. However, this finding was based on an experimental SARS-CoV-2 infection and therefore may not fully reflect natural conditions. The infected cats showed no signs of disease, indicating low viral transmission in cats. In a serology study on the cat population in Wuhan (initial epicenter Covid-19), 15 out of 102 cats were seropositive [13]. In the same study, three cats whose owners were Covid-19 positive showed the presence of high levels of SARS-CoV-2 neutralizing antibodies. However, the high titer of these antibodies was related to the fact that cats lived with individuals infected with Covid-19. The other six positive cats were either homeless or from cat clinics. Consequently, like humans, cats can also become infected with SARS-CoV-2 and trigger an immune response. It has also been found under experimental conditions that SARS-CoV-2 is transmitted among cats via the droplet pathway. Experimental studies revealed severe changes in the nasal cavity, tracheal mucosa and lungs in cats less than 3 months of age; however, the clinical signs of infection have not been described in detail. Nevertheless, an owner-infected cat with a positive Covid-19 result showed clear signs of diarrhea, vomiting, exertion, and dyspnoea.
This means that cats may not be easily infected with SARSCoV- 2 under natural conditions. Preliminary findings based on laboratory studies suggest that cats have a higher susceptibility to Covid-19 than all animal species tested. This is mainly because cats can become infected with the clinical disease and then spread the infection to other cats. Experimental evidence suggests that transmission of SARS-CoV-2 to cats from human Covid-19 positive patients occurs via the droplet route. It is worth noting that there is no evidence yet of zoonoses from cats to humans. While scientific evidence of a susceptibility of cats to Covid-19 is being gathered, there is an urgent need to thoroughly investigate the underlying patho-immune mechanism before drawing any conclusions. The possibility that pets are SARS-CoV-2 reservoirs cannot be ignored [14,15].
SARS-CoV-2 in mink
SARS-CoV-2 has been reported in mink reared on two separate farms in the Netherlands. Infected animals showing signs of respiratory failure have been associated with an increase in mortality. Following the SARS-CoV-2 outbreak on mink farms, authorities began mass culling, fearing that the mink could become a reservoir for the pandemic virus. The minks are suspected to have been infected by farm workers infected with Covid-19. However, preliminary epidemiological studies indicate that at least two farm workers have become infected by animal-to-human transmission by inhalation of dust and/or droplets containing the virus. This is the first report of animal-to-human transmission of SARS-CoV-2 [16].
SARS-CoV-2 among wild animals
SARS-CoV-2 related CoVs have been identified in Malay pangolins (Manis javanica) confiscated in southern China. The genetic sequences of the identified pangolin-associated CoVs showed strong similarity with the SARS-CoV-2 RBD and belonged to the SARS-CoV-2 related CoV subfamily. These findings suggest that pangolins may act as important hosts for emerging new CoVs such as SARS-CoV-2. Genetic and evolutionary studies have shown the presence of SARS-CoV-2-like CoV, which was named Pangolin- CoV, in dead Malayan pangolins [17]. Genomic analysis of genomic regions other than RBD indicates that pangolin CoV cannot be considered a direct source of SARS-CoV-2 and has not confirmed the possibility of pangolins as an intermediate host for SARS-CoV-2. The hypothesis that SARS-CoV-2 emerged directly from pangolins was rejected on the basis of two main findings: SARS-CoV-2 isolated from humans has a unique peptide insertion (PRRA) that plays a role in proteolytic cleavage of the spike protein. This RRAR fragment was absent from the pangolin coronavirus. It was also found that Pangolin-CoV are less similar to SARS-CoV-2 than the BetaCoV/bat/ Yunnan/RaTG13/2013 virus isolated from bats in Wuhan [18].
Tiger in: The National Veterinary Services Laboratories (NVSL) of the United States Department of Agriculture (USDA) have diagnosed SARS-CoV-2 in a tiger kept at the Bronx Zoo in New York. This tiger, along with other individuals of its species and lions, was tested immediately after showing symptoms of a respiratory disease. This was the first report of SARS-CoV-2 transmission from humans to wild animals. It is suspected that this Malayan tiger was infected by an asymptomatic guardian of animals with undiagnosed SARS-CoV-2 [19].
Covid-19 Chemotherapy
Currently, the world is focusing its attention on ensuring the availability of vaccines for humans (AstraZeneka, J&J, Moderna, Pfizer) to create herd immunity. Meanwhile, in Russia and the US, work is underway to develop the world’s first Covid-19 vaccine for companion animals. The vaccine, developed under the name Carnivac-Cov, has now undergone a clinical trial process in species such as dog, cat and mink. Tested for virus spread. The WSAVA believes that additional information about pets is needed before the vaccine can be given. In particular, studies should be carried out to confirm that administration of the vaccine reduces the disease and excretion of live SARS-CoV-2, and if so, the likely duration of immunity [20-22].
Access to vaccines in the world is increasing, and so is the percentage of the vaccinated human population. If we, as humans, maintain high standards of personal hygiene and prevent future infections, vaccination of companion animals may still be only a matter of debate. Otherwise, it is a mystery when it comes to livestock on fur farms, such as minks and sheds. The risk of infecting even single individuals on large-scale farms is enormous not only due to the health and safety of employees and residents of a given area, but also due to production costs. The introduction of vaccines on farms is being considered due to the serious ramifications of the current pandemic. Similar arguments have been suggested by scientists from the USA (Zoetis), who want to focus on animals when potentially vaccinating animals, threatened with extinction, when potentially vaccinating animals. These animals have a chance to become infected in national parks, in zoos, and to develop serious respiratory complications that they may not survive [21]; (Figure 1 & 2).
Figure 1: SARS-Cov-2 variants.
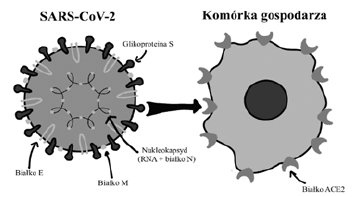
Figure 2: Schematic structure and binding of SARSCoV- 2 virus to the host cell. The SARS-CoV-2 virion consists of a nucleocapsid composed of the N protein and a single strand RNA, and a lipid-protein envelope containing the E, M and S proteins. The virus binds to the ACE2 protein via the S protein [22].
References
- (2020) Coronavirus: China's first confirmed covid 19 case traced back to November 17.
- (2020) The first COVID-19 case originated on November 17, according to Chinese officials searching for patient zero.
- Canrong Wu, Yang Liu, Yueying Y, Zhang P, Wu Zhong et al. (2020) Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharmaceutica Sinica B 10(5): 766-788.
- Guan Y, Zheng BJ, He YQ, Liu XL, Zhuang ZX, et al. (2003) Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science 302(5643): 276-278.
- Hemida MG, Perera RA, Wang P (2013) Middle East Respiratory Syndrome (MERS) coronavirus seroprevalence in domestic livestock in Saudi Arabia, 2010 to 2013. Euro Surveill 18(50): 20659.
- Wrapp D, Wang N, Corbett KS, Goldsmith JA, Lin Hsieh C, et al. (2020) Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367(6483): 1260-1263.
- Kuba K, Imai Y, Rao S (2005) A crucial role of Angiotensin Converting Enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 11(8): 875-879.
- Tang N, Li D, Wang X, Sun Z (2020) Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemostasis 18(4): 844-847.
- Danzi GB, Loffi M, Galeazzi G, Gherbesi E (2020) Acute pulmonary embolism and COVID-19 pneumonia: A random association? Eur Heart J 41(19): 1858.
- Goumenou M, Spandidos D, Tsatsakis A (2020) Possibility of transmission through dogs being a contributing factor to the extreme Covid-19 outbreak in North Italy. Mol Med Rep 21(6): 2293-2295.
- The government of the Hong Kong special administrative region-press releases. 2020b. Pet dog further tests positive for antibodies for COVID-19 virus.
- Chini M (2020) Coronavirus: Belgian cat infected by owner. The Brussels Times.
- Zhang Q, Zhang H, Huang K, Yang Y, Hui X, et al. (2020) SARSCoV-2 neutralizing serum antibodies in cats: A serological investigation.
- Morales RAJ, Dharma K, Sharun K, Tiwari R, Aldana BDK (2020) Susceptibility of felids to coronaviruses. Vet Rec 186(17): e21.
- Thomson GA (2020) COVID-19: Leaving lockdown-of Schrodinger, cats, testing and masks. Int J Clin Pract 74(8): e13519.
- Oreshkova N, Molenaar RJ, Vreman S, Harders F, Oude Munnink BB, et al. (2020) SARS-CoV-2 infection in farmed minks, the Netherlands, April and May 2020. Euro Surveill 25(23): 2001005.
- Lam TT, Shum MH, Zhu HC, Tong YG, Ni XB, et al. (2020) Identifying SARS-CoV-2 related coronaviruses in Malayan pangolins. Nature 583(7815): 286-289.
- Li X, Zai J, Zhao Q, Nie Q, Li Y, et al. (2020) Evolutionary history, potential intermediate animal host, and cross-species analyses of SARS-CoV-2. J Med Virol 92(6): 602-611.
- (2020) USDA statement on the confirmation of COVID-19 in a tiger in New York.
- Report of the world organization for animal health (OIE-World Organization for Animal Health) on animals affected by the SARS-CoV-2 virus.
- (2020) COVID-19-An update for WSAVA Members.
- Pawlik L, Śpiołek E, Fichna J, Tarasiuk A (2020) Characteristics of the SARS-CoV-2 virus and potential pharmacological treatment methods. Postepy Biochem 66(2): 83-90.
© 2021 Feliks Kania B. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






