- Submissions

Full Text
Developments in Anaesthetics and Pain Management
Transcranial Doppler (TCD) Ultrasonography and its Clinical Application-A Review and Update
Surjya Prasad Upadhyay1*, Piyush N Mallick2 and Waleed Elmatite3
1Specialist Anaesthesiologist, NMC Hospital, UAE
2Consultant Anaesthesiologist, Al Zahra Hospital Sharjah, UAE
3Fellow in Paediatric Anaesthesia, Women and child Hospital Buffalo, USA
*Corresponding author: Surjya Prasad Upadhyay, Specialist Anaesthesiologist, NMC Hospital, UAE
Submission: September 12, 2017;Published: June 07, 2018

ISSN 2640-9399 Volume1 Issue3
Abstract
Transcranial Doppler (TCD) Ultrasonography is a bedside, non-invasive duplex study of intracranial blood flow dynamic through some selected region or window in the cranial region. TCD provides information about the physiology of blood flow in the basal intracranial major arteries by measuring flow velocity and pulsality in different segment of these arteries. The physiologic data obtained from the cerebrovascular hemodynamic are complimentary to anatomical data obtained from various imaging techniques and it can be used as bedside monitor to assess the cerebrovascular changes in response to any intervention. Common application of TCD includes detection of vasospasm after subarachnoid haemorrhage, vasooclusion in sick cell disease, cerebral strokes, intracranial pressure monitoring, intraoperatively monitoring to detect micro-embolism, early detection of brain death and cerebral auto regulatory testing. The review focus on basic principles of TCD, scanning techniques and described application of TCD in some of the common clinical conditions.
Keyword: Transcranial Doppler Ultrasonography; Cerebral blood flow; Cerebral vasospasm; Sick cell disease; Intracranial pressure; Brain death; Cerebral auto regulation
Abbreviations: TCD: Transcranial Doppler; CBF: cerebral blood flow; PSV: peak systolic Velocities; EDV: End Diastolic Velocities; PI: Pulsatility Index ; RI: Resistive Index; MCA: middle cerebral artery; ACA: Anterior cerebral Artery; PCA: Posterior Cerebral Artery; BA: Basilar Artery; VA: Vertebral Artery; MAP: Mean Arterial Pressure; MFV: Mean Flow Velocity; ICP: Intra Cranial Pressure; NPV: Negative Predictive value
Introduction
Transcranial Doppler (TCD) Ultrasonography is a noninvasive technique that utilised a pulsed low-frequency ultrasonic transducer of low frequency through some selected region (window) in the cranial region to enable recording to flow velocity pattern in intracranial arteries. Unlike the cerebral angiography which provides an anatomical configuration of both extra and intracranial arteries and their smaller branches, TCD provides information about the physiology of blood flow in the basal intracranial major arteries by measuring flow velocity and pulsality in different segment of these arteries [1,2]. TCD being relatively inexpensive, non-invasive, reputable, portability and provides real time information that makes it more practical and convenience as bed side monitoring in intensive care setting for cerebral blood flow (CBF) velocity [3-5]. The physiologic data obtained from the cerebrovascular hemodynamic are complimentary to anatomical data obtained from various imaging techniques and it can be used as bedside monitor to assess the cerebrovascular changes in response to any intervention [4,5].
Aaslid et al. [6] in the year 1982 first demonstrated the technique of using pulsed Doppler ultrasound (without imaging facility) through transtemporal approach to measure the cerebral blood flow velocities [6]. The technique they used was nonduplex (non-imaging) or blind as there was no direct grey scale sonographic visualisation of cerebral arteries, the identity of the vessel being interrogated was determined by standard criteria that included transducer orientation, depth of the sample volume and direction of blood flow. This technique is still in use by many clinicians, however, inability to direct visualize target vessels and to perform angle correction, may have led to errors in the velocities recorded with this technique. The Power-motion mode Doppler or M-mode Doppler was described by Mark Moehring [7] in 2002 with 33 sampling gates to detect cerebral blood flow and cerebral embolism [7]. Modern TCD involves use of spectral Doppler and colour Doppler in addition to grey-scale tissue imaging that allows direct visualization of the major intra cranial arteries, allowing quick, accurate identification of arteries and their flow velocity dynamics.
Basic Principle
TCD Ultrasonography, like all Doppler imaging studies, is based on two main principles; the Doppler Effect and the Bernoulli principle. Sound wave with certain frequency when strikes a moving object, the frequency of the reflected sound wave is changes. The differences in the frequency between emitted and reflected waves is called “Doppler shift frequency” that is directly proportional to the speed of the moving object (blood flow in TCD). If the flow is towards the probe, results in shift to a higher frequency or positive Doppler shift and if the flow is away from the probe results in Doppler shift to lower frequency or negative Doppler shift. Since blood flow within a vessel is laminar, the Doppler signal obtained actually represents a spectral display of mixture of different Doppler shift from individual red blood cells [4].
The relationship between flow velocity and Doppler shift frequency is express by following formula
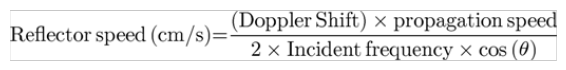
The propagation speed a sound wave in a particular medium, theta Θ is the angle of insonation relative to the direction of the blood vessel. The larger the angle of insonation, the larger is the cosine of the angle, and greater error in the measurement. If the angle is 0, (angle of insonation parallel to flow). We achieve the most accurate flow measurement since the cosine of 0 is 1. It is important to keep the angle below 30 degrees to keep the error below 15% (Figure 1).
Figure 2: Diagram shows the Doppler effect. Sound waves emitted at a specific frequency (fo) are reflected off moving red blood cells and back to the transducer at a higher or lower frequency (fr). The difference in frequencies, known as the Doppler shift, can be used to calculate the blood flow velocity (V) and direction. θ = angle between the incident ultrasound beam and the direction of blood flow.
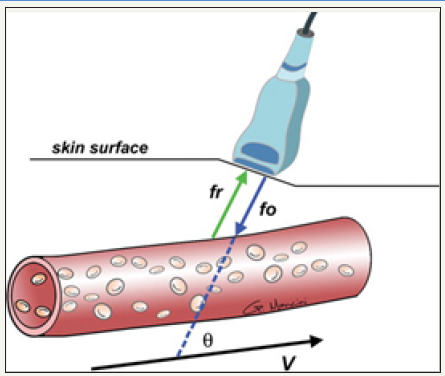
Figure 3: Diagram of the Bernoulli principle shows that as fluid flows from a conduit or vessel of greater diameter to one of lesser diameter, the velocity of flow increases and the pressure decreases to allow the same volume of fluid to pass through the narrower area.
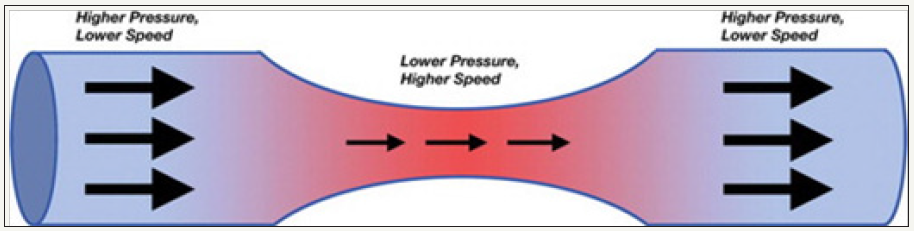
The Bernoulli principle described the flow in terms of velocity and pressure of liquid (blood) through different size of conduit (vessels). Blood flow velocity increases and pressure decreases when blood flow from a greater to lesser diameter (vasospasm), this is to allow the same volume of blood pass through narrow area. The changes in blood velocity (detection of vasospasm) can be estimated using Doppler Effect and Doppler equation (Figure 2).
TCD relies on pulse wave Doppler to image a particular vessel and a received echo generates an electrical impulse which is processed electronically to produce a spectral waveform [8,9]. A spectral Doppler waveform is visual display of flow-velocities within a specific are of blood vessels as a time velocity curve. Spectral analysis can thus be use to estimate not only the blood flow velocity, it can also used to calculate the peak systolic (PSV) and end diastolic (EDV) velocities, systolic upstroke or acceleration time, pulsatility index (PI), resistive index (RI) and time averaged mean maximum velocity (V mean) (Figure 3). TCD devices are equipped with larger sample volume unlike the normal Pulse Wave Doppler (PWD) for other uses; this is to obtain better quality of signal in noisy background also. Standard TCD is recorded bilaterally using PWD for at least 10 cardiac cycles [10].
PI: Pulsatility Index; TCD: Transcranial Doppler
Figure 4: Duplex Doppler image and spectral waveform show the velocity of blood flowing within the PCA. The PSV, EDV, MFV, and resistive index (RI) are automatically calculated from the waveform.
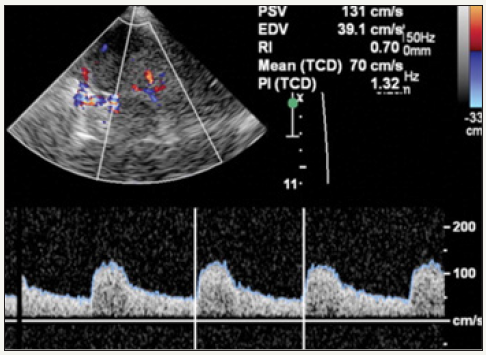
Image Technique
The TCD Ultrasonography examination is performed by using phase array probe of 1-2MHz .The higher frequency waves have lower penetration and not able to penetrate the through the skull bone in adults and hence are not useful for intracranial scanning [8,9]. Based on anatomical location of the major cerebral arteries and ease of ultra sound penetration into brain though thinner region of skull has been identified, termed as acoustic window [8,9].
Four Acoustic Windows Are Used For TCD
Figure 5: Color Doppler flow image obtained with a transtemporal window shows blood flow through the circle of Willis.
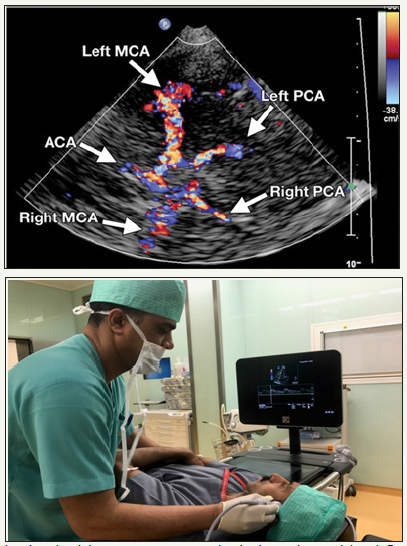
Transtemporal, transformational or sub occipital, Transorbital and submandibular (retromandibular). Each window specifically target particular portion of major branch of the circle of Willis, which are first optimally scan using grey scale and colour flow imaging; once a particular vessel is identified, spectral waveform are obtained using pulse wave Doppler [3,8-10]. The most commonly used acoustic window is transtemporal window, located above the zygomatic ridge between the lateral can thus of the eye and auricular pinna, through this window all three major arteries (anterior, middle and posterior cerebral arteries) forming the circle of Willis as well as terminal portion of Internal carotid artery can be insonated (Figure 4). once probe is placed in the temporal window, identification of sphenoid bone, through the butterfly wing sign guide the visualization of the middle cerebral artery (MCA) in almost all patients which usually lies at depth of 50-65mm indicated by red colour signal as flow is towards the probe, with fine adjustment and following the flow pattern, the branching of MCA can be identified. The internal carotid artery (ICA) bifurcation observed at about 55- 70mm in adults and is represented by bidirectional flow (red and blue in colour Doppler), the Anterior cerebral artery (ACA) flow is away from the probe (blue) (Figure 4).
Once signal from MCA and ACA are obtained, the ultrasound probe is slowly oriented posteriorly by 10-30 degrees, initially there will be a flow gap before signals from the posterior cerebral artery (PCA) is obtained at 55-70mm. Two segment of PCA are obtained, P1 represented by flow towards the probe and P2 away from the probe.
Figure 6: Color Doppler flow image obtained with a Transformational or sub occipital window shows the vessels of the posterior cerebral circulation.

Transformational or sub occipital approach involve placing the transducer in sub occipital region in a flex neck, in mid line below the occipital, medial to the mastoid process and the probe is angled or tilted towards the bridge of the nose or contra lateral eye to insonate portion of the vertebral artery (VA) at a depth of 50-75mm (flow away from the probe) and further upward and medial tilting of probe is done to obtained from the basilar artery (BA) at a depth of 75-110mm (Figure 5).
Figure 7: Submandibular and Transorbital windows.
Transorbital approach involves placing Ultrasonography probe gently on the closed eye lid to visualize the ophthalmic artery and the cavernous portion of the internal carotid artery (carotid siphon). The probe is angled slightly medially and upward, at around 60 mm ophthalmic artery can be traced followed by trace from the carotid siphon. Sub mandibular window is at just below the angle of jaw and can be used to locate the distal extra cranial portion of internal carotid artery in the neck (Figure 6).
Target artery is identified depending on the appropriate acoustic window, probe orientation and angle, depth of insonation and direction of flow, in difficult scenario when it is not possible to differentiate the anterior from posterior artery, dynamic maneuvers such as direct carotid compression or vibration and resulting waveform changes are recorded [3,9].
TCD Indices
Peak Systolic Velocity (PSV) -The maximum value of flow velocity in systole, at the apex of the waveform.
End Systolic Velocity (EDV)-The velocity measured at end diastole, usually at the lowest point before a new waveform begins. The central parameter in TCD is the mean flow velocity (MFV) which can be calculated similar to that of mean arterial pressure (MAP), the MFV is equal to 1/3rd PSV + 2/3rd EDV. (MFV= {PSV+EDV x 2}/3). The MFV can be influenced by number of physiological factors [11-13] (Table1).
Table 1: Physiological Factors Influencing MFV.

Common pathological factors increasing MFV are vasospasm, stenosis, hyper dynamic flow, whereas, hypotension, decrease in cerebral blood flow, intracranial pressure or brain stem death [11] other important TCD indices are:
Gosling’s pulsatility index (PI)
This is the most commonly used TCD parameter to determine the flow resistance. PI is the measure of pulsatility of blood flow that reflects the resistance to blood flow. It is the difference between systolid and diastolic flow velocity divided by mean velocity. It is calculated as PI= (PSV-EDV)/MFV. Normal value-0.5-1.19. Downstream obstruction will increase the PI and proximal obstruction or stenosis may decrease the PI due to down stread vasodilatation [14,15]. Also it is affected by change in Intra Cranial Pressure (ICP) in a linear way and a progressive rise in PI may reflect rise in ICP [16,17]. Patients with Arterio-Venous (AV) malformation will have reduced PI as the resistance to flow suddenly reduced due to distal venous flow [18].
Pourcelot resistivity index (RI)
It is similar to pulsatility index. It measures the resistance to blood flow distal to the site of measurement. It is calculated as RI= (PSV-EDV)/PSV; value greater than 0.8 indicates increased in downstream resistance [11].
Lindegaard ratio (LR)
It’s a ration of mean flow velocity in intracranial to extra cranial carotid artery and calculated as MFV in Middle cerebral artery/MFV in extra cranial internal carotid artery [19]. The LR differentiates between hyper dynamic flows from vasospasm. LR more than 3 indicates cerebral vasospasm. A high LR ratio indicates increased in intracranial flow due to vasospasm and abnormally low LR indicates hyper dynamic flows like in A-V malformation [20]. A modified LR ratio for intracranial bsilar and extra cranial vertebral artery can also be cam also be used to calculate vasospasm in posterior circulation [21].
Micro embolic signal (MES) detection
TCD a useful monitor intraoperatively for carotid and other cardiac surgery where there is high risk of micro embolism [22,23].
Figure 8: Abnormal systolic acceleration and accelerated flow velocity with flow turbulence (bruit) indicated by arrow.

Abnormal spectrum waveforms
Delayed systolic flow acceleration or flattened systolic upstroke and slow diastolic deceleration with end diastolic velocity (EDV) above 50% of peak systolic velocity (PSV) is hallmark of stenosis proximal to the site of PWD this is known as blunted flow, and is due to compensatory vasodilatation distal to the stenosis (Figure 7). Patients with focal intracerebral stenosis/ vasospasm- there will be focal flow acceleration, with increasing stenosis; the increased in the flow creates flow turbulence, represented by bruit, which is seen as symmetrical artefact on either side of baseline.
Clinical Application of TCD
Table 2: Common Clinical Applications of TCD.

TCD was initially used to diagnose vasospasm following subarachnoids haemorrhage. However, a clinical use of TCD has expanded greatly over the decades and it has emerged as bedside, non-invasive tool for evaluating cerebral artery patency, detection of stenosis, embolism, flow pattern and even as alternative monitoring of ICP. Common clinical applications of TCD can be summarised as categorical groups (Table 2).
Monitoring of vasospasm in subarachnoid haemorrhage (SAH)
TCD was initially invented for monitoring of cerebral vasospasm after SAH [6,7]. The basis of using TCD in vasospasm is on Bournellis principle, the narrowing of vessel will lead to increase in velocity of blood flow, even though absolute flow decreased. TCD is a reliable, cost effective tool for monitoring of vasospasm after SAH delayed cerebral vasospasm is one of the common complication after SAH, can occur in up to 70% between 4-17 days, which can leads to delayed ischemic deficit in up 25% patients [24-26]. There are reports of early vasospasm within 48 hours in up to 13% cases, [27- 32] and much delayed vasospasm even after 20 days; morbidity and mortality are considered to increase significantly, longer the period of vasospasm [33]. Use of TCD can be used to early detection of even asymptomatic vasospasm, follow progression of vasospasm and can be used to monitor the effect of treatment or intervention for vasospasm [34-39].
Suggested protocol for TCD in SAH by Sharma et al [38]:
- First baseline TCD as soon as possible after the diagnosis of SAH. Flow velocity to be obtained from both extra cranial and intracranial artery to calculate the LR index.
- Daily or more frequently if needed evaluation by TCD for early detection of vasospasm.
- Standard diagnostic criteria to be used for diagnosis of Vasospasm (Table 2).
- If TCD suggested of vasospasm, to initiate treatment for vasospasm and TCD re-evaluation every 6-8 hours.
- Information regarding ICP if available should be taken into account during interpretation of TCD.
- In doubtful finding, other diagnostic modality (imaging) should be employed.
TCD in comparison to angiography has good sensitivity and specificity in diagnosis vasospasm for both MCA and VA. [35,40,41] Mean flow velocity (MFV) of MCA greater than 120 cm/sec has 99% specificity and 67% sensitivity to identify angiographic vasospasm of 25% or more [41]. The positive predictive value (PPV) increases to 98% when MFV exceed more than 200 cm/sec. whereas, when the MFV less than 12 cm/sec has 94% negative predictive value (NPV) [42]. Therefore, MFV of more than 200 cm/s or less than 120 cm/sec could predict both present and absence of MCA vasospasm, respectively. Unlike the sensitivity and specificity of even in mild vasospasm of MCA, TCD is little weaker in detecting less than 50% narrowing in VA and BA [35,40,43,44].
Similarly, ACA and PCA vasospasm has lower specificity and sensitivity in mild to moderate vasospasm unlike the MCA vasospasm [45]. It is daily changes in MFV that is more important than the absolute value; an increase in MFV is highly predictive of vasospasm. MFV rise of 50cm/sec or more within 24 hours or MFV increases of more than 65cm/s per day from day 3-7 indicate development of vasospasm and high risk for delayed cerebral ischemia [46,47].Though the TCD is a valid diagnostic tool to identify and to monitor the development of intra cerebral vasospasm after SAH. The interpretation of TCD finding should be correlated with clinical assessment and other diagnostic tools like computed angiography should be used for diagnosis of vasospasm after SAH instead of the single independent test [47,48].
Acute ischemic/embolic stroke
TCD can accurately identify acute occlusions of intracranial arteries with high, specificity, sensitivity, PPV, and NPV of greater than 90% for MCA and 70-90% for ICA siphon, VA and BA [49]. TCD has been compared with Magnetic resonance angiography (MRA) and Computed tomography angiography (CTA) in early evaluation in acute ischemic strokes. It has been used to assess steno-occlusive pathology of terminal ICA, ICA siphon and MCA. TCD is almost 100% specific and 93% sensitive for identifying the MCA ischemia, whereas MRA had 74% specificity and only 46% sensitivity in the assessment of Intracerebral arteries. Moreover, TCD can be used in emergency department as bed side tool and provide real time information about cerebral blood flow (CBF) [50-52].
In addition to diagnostic tool, TCD has more prognostic value in strokes. A complete arterial occlusion at presentation predicted worse neurologic outcome, disability and death after 90 days, [53,54] a normal TCD finding predicted early neurological improvement [47,55]. Unlike other imaging modality, the advantages of TCD are that it can be used serially to detect any cerebral hemodynamic changes that would otherwise go undetected by a single MRA, CTA studies [56].
TCD is also useful to assess the hemodynamic changes before and after thrombolysis; recanalisation within 6 hours of symptoms onset or within 30 min of thrombolysis is associated with marked clinical improvement and functional independence at 3 month [57,58]. TCD can also be useful tool to detect early re occlusion after thrombolysis.
TCD in Sickle-Cell Disease
Patients with sickle cell disease (SCD) (homozygous) carry a significant risk of cerebral strokes, 11% all SCD children developed strokes before the age of 20 years and majority of these strokes involve occlusion or stenosis of the distal portion of ICA or proximal portion of MCA [38]. Pathophysiologies involve increasing circulation of irreversibly sickled cells and their adherence to the vascular endothelium which leads to vaso-occlusion and ischemia [59].
TCD is a useful tool in assessment of children with SCD to identify the risk of stroke, need of blood transfusion. Elevated blood flows in SCD are related to anemia, cerebral vasodilatation caused by tissue hypoxia or vessel stenosis. Blood transfusion correct some of these abnormalities to an extent and reduces cerebral blood flow velocity and thereby the risk of stroke [60,61]. Detection of time average MFV exceeding 200cm/s on two separate occasion is the main risk factor for the first-ever stroke [62,63]. This can be preventable up to 90% by timely transfusion blood [63]. Stroke prevention in sickle cell disease (STOP) trial has developed a protocol for TCD screening in children with SCD. The STOP protocol is different than routine clinical TCD examination, where they uses time-average mean of the maximum (TAMM) velocities of ICA and or MCA recorded on two separate occasions at least 2 weeks apart [64]. The detail description of STOP protocol is beyond the scope of this chapter (Table 3).
Table 3: STOP classification for risk stratification and treatment strategy [64].

Traumatic brain injury (TBI) and raised ICP
TBI have spectra of effects on brain and cerebral circulation, immediately after trauma, there may be hypo perfusion (day 0) followed by hyperaemia (day1-3), there may be delayed vasospasm and raised ICP after the initial phase is over [65]. TCD as a noninvasive tool can be used to early detection of cerebral hemodynamic changes, it also provide long term prognostic information. TCD derived pulsatility index (PI) provides useful information about ICP and CPP, avoiding complications of invasive monitoring [16,17,66] Recently Wakerley et al. [67], conducted a study to define the relationship of PI and ICP, they performed TCD measurement and simultaneously Lumbar puncture for ICP measurement. TCD-PI has acceptable accuracy in differentiating patients with ICP more than 20cm of cerebrospinal fluid (CSF). Serial measurement over short period of time are reliable than single measurement [67]. There is no standardisation technique or formula for absolutely quantifying the ICP/ CPP by TCD method, hence at present, TCD is reserved for assessing serial change, rather than absolute value.
TCD monitoring for spontaneous emboli
Emboli from carotid plaque or from cardiac origin (left atrial thrombus or right to Left shunt) can produce cerebral ischemia by blocking regional perfusion. Extended monitoring of intracranial artery distal to embolic origin site can detect spontaneous microemboli called Micro-embolic signals (MES).
The International Cerebral Hemodynamic Society described MES as [38]
- Random occurrence during the cardiac cycle.
- Brief duration (usually <0.1 s).
- High intensity (>3 dB over background).
- Primarily unidirectional signal (if fast Fourier transform is used).
- Audible component (chirp, whistle, or pop).
Patients with history of cerebrovascular ischemia or high grade carotid stenosis can also undergo TCD monitoring for MES [68-70]. MES can also be used to assess the treatment effectiveness after initiating anti platelet drug therapy in carotid stenosis [71,72].
Right to left (R-L) shunt detection
Patent foramen ovale can be present in approximately 25% of general population and these patients are at risk of paradoxical embolism [73]. TCD bubble test can be done to detect R-L shunt in patients with cerebral ischemia due to suspected paradoxical embolism. TCD is considered to be superior to transesophageal echocardiography as it has not only higher sensitivity and specificity for detection of R-L shunt, it can quantify its functional -potential for embolism [74,75].
TCD bubble teast involve injection of small quantity of micro bubbles, produced by agitation with saline and patient own blood through antecubutal vein and simultaneous TCD scanning for detection of MES in one MCA. The test is repeated with change in body posture.
The R-L shunt can be quantified by international consensus criteria [76]
- Grade 0: No MES.
- Grade 1: MES count 1-10.
- Grade 2: MES count 11-30.
- Grade 3: MES count 31-100.
- Grade 4: MES count 101-300.
- Grade 5: MES count more than 300.
TCD in brain death: Brain stem dysfunction and death is a clinical diagnosis based on clinical evaluation and various provocative test and electroencephalography. However, the diagnosis of brain stem death may be delayed considerably in condition like use of sedative and muscle relaxant, metabolic derangement, hypothermia. TCD may be alternative confirmatory test in such situation. This phenomenon could have serious implications in cases where organ preservation is needed in preparing for possible organ transplant [77-80]. TCD per cannot be used on its own to diagnose brain death since this is a clinical diagnosis. TCD can be used to help detecting the cerebral circulatory changes. A prolonged (more than 30 min) absence of cerebral perfusion or cerebral circulatory arrest is detected using one of the following criteria [81].
- Reverberating (oscillating) flow pattern- an oscillating waveform- with reversal of equal flow pattern in systole and diastole- zero net flow.
- Small systolic spike less than 200ms duration of less than 50cm/sec peak systolic velocity with absent diastolic flow.
- Absence flow signals in intracranial circulation with typical flow signal in extra cranial circulation.
Cerebral auto-regulation or vasomotor reactivity
Cerebral auto regulation refers to the maintenance of cerebral blood flow (CBF) despite changes in cerebral perfusion pressure over the range of 50-150mm/hg [82]. Impairment in auto regulatory response has been demonstrated in varieties of brain insult such as strokes, traumatic brain injury, menigo-encephalitis, carotid disease etc. Impaired cerebral auto regulation or vasomotor response has prognostic value. TCD can be used to assess the various aspects of CBF regulation in response to change in blood pressure, circulating level of vasomotor in a dynamic way [82-84]. A pressure reactivity index Prx and CBF related index has been introduced to assess the cerebral auto regulation [85] but none of the index has been considered as gold standard [86].Changes in MFV in MCA is used as surrogate for changes in CBF. Many different non-pharmacologic techniques such as direct carotid artery compression, Valsalva manoeuvre, application of PEEP, head up tilting, negative pressure to lower body portion etc were used to induce pressure changes [87-90].
The TCD assessment of cerebral auto regulation is still an area of clinical research given the high temporal resolution, convenience and non-invasiveness of the technique. Significant auto regulatory impairment in TBI and strokes are common and are associated with poor neurologic outcome. However, the role of auto regulatory assessment in carotid stenosis, syncope or extra cranial pathology is less clear.
Conclusion
TCD being non-invasive, portability and provide real time information and high temporal resolution have promoted its use as bedside neuro monitoring tool. Majority of its use are early detection of impaired cerebral flow dynamic and it helped in initiation of preventive measures in SAH, SCD, TBI and Strokes or even preparation of organ retrieval in impending brain death. Advanced application of TCD includes evaluating various mechanism of cerebral ischemia. TCD helps in planning and monitoring the cerebral hemodynamic, effectiveness of therapy as well as ids in establishing the prognosis. The main limitation of TCD is high operator dependency and it requires some technical expertise and training to standardise its application.
References
- American Academy of Neurology Assessment: Transcranial Doppler (1990) Report of the American Academy of Neurology, Therapeutics and Technology Assessment Subcommittee. Neurology 40(4): 680-681.
- Newell DW (1994) Transcranial Doppler ultrasonography. Neurosurgery Clinics of North America 5(4): 619-631.
- Moppett IK and Mahajan RP (2004) Transcranial Doppler ultrasonography in anaesthesia and intensive care. Br J Anaesth 93(5): 710-724.
- Lupetin AR, Davis DA, Beckman I, Dash N (1995) Transcranial Doppler sonography Part I: Principles, techniques and normal appearances. Radiographics 15(1): 179-191.
- Lupetin AR, Davis DA, Beckman I, N Dash (1995) Transcranial Doppler sonography Part II: Evaluation of intracranial and extracranial abnormalities and procedural monitoring. Radiographics 15(1): 193- 209.
- Aaslid R, Markwalder TM, Nornes H (1982) Noninvasive Transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg 57(6): 769-774.
- The modern Power-motion mode Doppler or M-mode Doppler was described by Mark Moehring in 2002 with 33 sampling gates to detect cerebral blood flow and cerebral embolism p. 7.
- Tegeler CH, Ratanakom D (1999) Physics and principle. In: Babikian VL and Wechsler LR (Eds.), Transcranial Doppler Ultrasonography. Waltham MA: Butterworth-Heinemann, Oxford, UK, p. 3-11.
- Nicoletto HA, Burkman MH (2009) Transcranial Doppler series part II: performing a transcranial Doppler. Am J Electroneurodiagnostic Technol 49(1): 14-27.
- D’Andrea A, Conte M, Scarafile R, Lucia Riegler, Rosangela Cocchia, et al. (2016) Transcranial Doppler Ultrasound: Physical Principles and Principal Applications in Neurocritical Care Unit. J Cardiovasc Echogr 26(2): 28-41.
- White H, Venkatesh B (2006) Applications of transcranial Doppler in the ICU: a review. Intensive Care Med 32(7): 981-994.
- Schatlo B, Pluta RM (2007) Clinical applications of Transcranial Doppler sonography. Rev Recent Clin Trials 2(1): 49-57.
- Nicoletto HA, Burkman MH (2009) Transcranial Doppler series part III: interpretation. Am J Electroneurodiagnostic Technol 49(3): 244-259.
- Gosling RG, King DH (1974) Arterial assessment by Doppler shift ultrasound. Proc R Soc Med 67(1 Pt 6): 447-449.
- Nicoletto HA, Burkman MH (2009) Transcranial Doppler series part IV: case studies. Am J Electroneurodiagnostic Technol 49(4): 342-360.
- Homburg AM, Jakobsen J, Enevoldsen E (2009) Transcranial Doppler recordings in raised intracranial pressure. Acta Neurol Scand 87(6): 488-493.
- Bellner J, Romner B, Reinstrup P, Kristiansson KA, Ryding E, et al. (2004) Transcranial Doppler Sonography Pulsatality Index (PI) reflects Intracranial Pressure (ICP). Surg Neurol 62(1): 45-51.
- Nicoletto HA, Burkman MH (2009) Transcranial Doppler series part IV: case studies. Am J Electroneurodiagnostic Technol 49(4): 342-360.
- Lindegaard KF, Nornes H, Bakke SJ, Sorteberg W, Nakstad P (1988) Cerebral vasospasm after subarachnoid haemorrhage investigated by means of transcranial Doppler ultrasound. Acta Neurochir Suppl (Wien) 42: 81-84.
- Aaslid R, Huber P, Nornes H (1984) Evaluation of cerebrovascular spasm with transcranial Doppler ultrasound. J Neurosurg 60(1): 37-41.
- Topcuoglu MA (2012) Transcranial Doppler ultrasound in neurovascular diseases: diagnostic and therapeutic aspects. J Neurochem 123(Suppl 2): 39-51.
- King A, Markus HS (2009) Doppler embolic signals in cerebrovascular disease and prediction of stroke risk: a systematic review and metaanalysis. Stroke 40(12): 3711-3717.
- Ogasawara K, Suga Y, Sasaki M, Chida K, Kobayashi M, et al. (2008) Intraoperative Microemboli and Low Middle Cerebral Artery Blood Flow Velocity Are Additive in Predicting Development of Cerebral Ischemic Events After Carotid Endarterectomy. Stroke 39(11): 3088-3091.
- Vergouwen MD, Vermeulen M, van Gijn J, Rinkel GJ, Wijdicks EF, et al. (2010) Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 41(10): 2391-2395.
- Dorsch NA (2011) clinical review of cerebral vasospasm and delayed ischemia following aneurysm rupture. Acta Neurochir Suppl 110(Pt 1): 5-6.
- Papaioannou V, Dragoumanis C, Theodorou V, Konstantonis D, Pneumatikos I, et al. (2008) Transcranial Doppler ultrasonography in intensive care unit. Report of a case with subarachnoid hemorrhage and brain death and review of the literature. Greek E J Med 6: 95-104.
- Zubkov AY, Rabinstein AA (2009) Medical management of cerebral vasospasm: Present and future. Neurol Res 31(6): 626-631.
- Smith M (2007) Intensive care management of patients with subarachnoid haemorrhage. Curr Opin Anaesthesiol 20(5): 400-407.
- Dorsch NW, King MT (1994) A review of cerebral vasospasm in aneurysmal subarachnoid haemorrhage Part I: Incidence and effects. J Clin Neurosci 1(1): 19-26.
- Mascia L, Fedorko L, terBrugge K, Filippini C, Pizzio et al. (2003) The accuracy of transcranial Doppler to detect vasospasm in patients with aneurysmal subarachnoid hemorrhage. Intensive Care Med 29(7): 1088-1094.
- Otten ML, Mocco J, Connolly ES, Jr, Solomon RA (2008) A review of medical treatments of cerebral vasospasm. Neurol Res 30(5): 444-449.
- Marshall SA, Nyquist P, Ziai WC (2010) The role of transcranial Doppler ultrasonography in the diagnosis and management of vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurg Clin N Am 21(2): 291-303.
- Harders AG, Gilsbach JM (1987) Time course of blood velocity changes related to vasospasm in the circle of Willis measured by transcranial Doppler ultrasound. J Neurosurg 66(5): 718-728.
- Sloan MA, Haley EC Jr, Kassell NF, Henry ML, Stewart SR, et al. (1989) Sensitivity and specificity of transcranial Doppler Utrasonography in the diagnosis of vasospasm following subarachnoid hemorrhage. Neurology 39(11): 1514-1518.
- Sloan MA, Burch CM, Wozniak MA, Rothman MI, Rigamonti D, et al. (1994) Transcranial Doppler detection of vertebrobasilar vasospasm following subarachnoid hemorrhage. Stroke 25(11): 2187-2197.
- Nakae R, Yokota H, Yoshida D, Teramoto A (2011) Transcranial Doppler ultrasonography for diagnosis of cerebral vasospasm after aneurysmal subarachnoid hemorrhage: mean blood flow velocity ratio of the ipsilateral and contralateral middle cerebral arteries. Neurosurgery 69(4): 876-883.
- Rawal S, Barnett C, John Baptiste A, Thein HH, Krings T, et al. (2015) Effectiveness of diagnostic strategies in suspected delayed cerebral ischemia: a decision analysis. Stroke 46(1): 77-83.
- Sharma AK, Bathala L, Batra A, Mehndiratta MM, Sharma VK (2016) Transcranial Doppler: Techniques and advanced applications: Part 2. Ann Indian Acad Neurol 19(1): 102-107.
- McGirt MJ, Blessing RP, Goldstein LB (2003) Transcranial Doppler monitoring and clinical decision-making after subarachnoid hemorrhage. J Stroke Cerebrovasc Dis 12(2): 88-92.
- Soustiel JF, Shik V, Shreiber R, Tavor Y, Goldsher D (2002) Basilar vasospasm diagnosis: Investigation of a modified “Lindegaard Index” based on imaging studies and blood velocity measurements of the basilar artery. Stroke 33(1):72-77.
- Lysakowski C, Walder B, Costanza MC, Tramèr MR (2001) Transcranial Doppler versus angiography in patients with vasospasm due to a ruptured cerebral aneurysm: A systematic review. Stroke 32(10): 2292- 2298.
- Vora YY, Suarez Almazor M, Steinke DE, Martin ML, Findlay JM (1999) Role of transcranial Doppler monitoring in the diagnosis of cerebral vasospasm after subarachnoid haemorrhage. Neurosurgery 44(6): 1237-1247.
- Harders A, Gilsbach J (1985) Transcranial Doppler sonography and its application in extracranial-intracranial bypass surgery. Neurol Res 7(3): 129-141.
- Skjelland M, Krohg Sørensen K, Tennøe B, Bakke SJ, et al. (2009) Cerebral microemboli and brain injury during carotid artery endarterectomy and stenting. Stroke 40(1): 230-234.
- Wozniak MA, Sloan MA, Rothman MI, Burch CM, Rigamonti D, et al. (1996) Detection of vasospasm by transcranial Doppler sonography. The challenges of the anterior and posterior cerebral arteries. J Neuroimaging 6(2): 87-93.
- Frontera JA, Fernandez A, Schmidt JM, Claassen J, Wartenberg KE, et al. (2009) Defining vasospasm after subarachnoid hemorrhage: What is the most clinically relevant definition?. Stroke 40(6): 1963-1968.
- Sloan MA, Alexandrov AV, Tegeler CH, Spencer MP, Caplan LR, et al. (2004) Assessment: Transcranial Doppler ultrasonography: report of the therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 62(9): 1468-1481.
- Gonzalez NR, Boscardin WJ, Glenn T, Vinuela F, Martin NA (2007) Vasospasm probability index: a combination of transcranial Doppler velocities, cerebral blood flow, and clinical risk factors to predict cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg 107(6): 1101-1112.
- Connolly ES, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Higashida RT, et al. (2012) Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 43(6): 1711-1737.
- Demchuk AM, Christou I, Wein TH, Felberg RA, Malkoff M, et al. (2000) Accuracy and criteria for localizing arterial occlusion with transcranial Doppler. J Neuroimaging 10(1): 1-12.
- Razumovsky AY, Gillard JH, Bryan RN, Hanley DF, Oppenheimer SM (1999) TCD, MRA and MRI in acute cerebral ischemia. Acta Neurol Scan 99(1): 65-76.
- Tsivgoulis G, Sharma VK, Lao AY, Malkoff MD, Alexandrov AV (2007) Validation of transcranial Doppler with computed tomography angiography in acute cerebral ischemia. Stroke 38(4): 1245-1249.
- Camerlingo M, Casto L, Censori B, Servalli MC, Ferraro B, et al. (1996) Prognostic use of Ultrasonography in acute non hemorrhagic carotid stroke. Ital J Neurol Sci 17(3): 215-218.
- Baracchini C, Manara R, Ermani M, Meneghetti G (2000) The quest for early predictors of stroke evolution: Can TCD be a guiding light? Stroke 31(12): 2942-2947.
- Kushner MJ, Zanette EM, Bastianello S, Mancini G, Sacchetti ML, et al. (1991) Transcranial Doppler in acute hemispheric brain infarction. Neurology 41(1): 109-113.
- Akopov S, Whitman GT (2002) Hemodynamic studies in early ischemic stroke: serial transcranial Doppler and magnetic resonance angiography evaluation. Stroke 33(5): 1274-1279.
- Christou I, Alexandrov AV, Burgin WS, Wojner AW, Felberg RA, et al. (2000) Timing of recanalisation after tissue plasminogen activator therapy determined by transcranial Doppler correlates with clinical recovery from ischemic stroke. Stroke 31(8): 1812-1816.
- Alexandrov AV, Burgin WS, Demchuk AM, El Mitwalli A, Grotta JC (2001) Speed of intracranial clot lysis with intravenous tissue plasminogen activator therapy: Sonographic classification and short-term improvement. Circulation 103(24): 2897-2902.
- Platt OS (2006) Prevention and management of stroke in sickle cell anaemia. Hematology Am Soc Hematol Educ Program pp. 54-57.
- Venketasubramanian N, Prohovnik I, Hurlet A, Mohr JP, Piomelli S (1994) Middle cerebral artery velocity changes during transfusion in sickle cell anaemia. Stroke 25(11): 2153-2158.
- Russell M, Goldberg H, Hodson A, Kim HC, Halus J, et al. (1984) Effect of transfusion therapy on arteriographic abnormalities and on recurrence of stroke in sickle cell disease. Blood 63(1): 162-169.
- Adams R, McKie V, Nichols F, Carl E, Zhang DL, et al. (1992) The use of transcranial ultrasonography to predict stroke in sickle cell disease. N Engl J Med 326(9): 605-610.
- Adams RJ, McKie VC, Hsu L, Files B, Vichinsky E, et al. (1998) Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med 339(1): 5-11.
- Lee MT, Piomelli S, Granger S, Miller ST, Harkness S (2006) Stroke Prevention Trial in Sickle Cell Anaemia (STOP): extended follow-up and final results. Blood 108(3): 847-852.
- Martin NA, Patwardhan RV, Alexander MJ, Africk CZ, Lee JH, et al. (1997) Characterization of cerebral hemodynamic phases following severe head trauma: hypoperfusion, hyperemia, and vasospasm. J neurosurgery 87(1): 9-19.
- Saqqur M, Zygun D, Demchuk A (2007) Role of Transcranial Doppler in neurocritical care. Crit Care Med 35(5 suppl): S216-223.
- Wakerley BR, Kusuma Y, Yeo LL, Liang S, Kumar K, et al. (2015) Usefulness of transcranial Doppler-derived cerebral hemodynamic parameters in the non invasive assessment of intracranial pressure. J Neuroimaging 25(1): 111-116.
- Yeo LL, Sharma VK (2010) Role of transcranial Doppler ultrasonography in cerebrovascular disease. Recent Pat CNS Drug Discov 5(1): 1-13.
- Spencer MP, Thomas GI, Nicholls SC, Sauvage LR (1990) Detection of middle cerebral artery emboli during carotid endarterectomy using Transcranial Doppler ultrasonography. Stroke 21(3): 415-423.
- King A, Markus HS (2009) Doppler embolic signals in cerebrovascular disease and prediction of stroke risk: A systematic review and metaanalysis. Stroke 40(12): 3711-3717.
- Markus HS, Droste DW, Kaps M, Larrue V, Lees KR, et al. (2005) Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using doppler embolic signal detection: The Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CARESS) trial. Circulation 111(17): 2233-2240.
- Wong KS, Chen C, Fu J, Chang HM, Suwanwela NC, et al. (2010) Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): A randomised, open-label, blinded endpoint trial. Lancet Neurol 9(5): 489-497.
- Cabanes L, Mas JL, Cohen A, Amarenco P, Cabanes PA, et al. (1993) Atrial septal aneurysm and patent foramen ovale as risk factors for cryptogenic stroke in patients less than 55 years of age. A study using transesophageal echocardiography. Stroke 24(12): 1865-1873.
- Jauss M, Kaps M, Keberle M, Haberbosch W, Dorndorf W (1994) A comparison of transesophageal echocardiography and Transcranial Doppler sonography with contrast medium in the detection of patent foramen ovale. Stroke 25(6): 1265-1267.
- Belvís R, Leta RG, MartíFàbregas J, Cocho D, Carreras F, et al. (2006) Almost perfect concordance between simultaneous transcranial Doppler and transesophageal echocardiography in the quantification of right to left shunts. J Neuroimaging 16(2): 133-138.
- Lao AY, Sharma VK, Tsivgoulis G, Frey JL, Malkoff MD, et al. (2008) Detection of right-to-left shunts: Comparison between the international consensus and spencer logarithmic scale criteria. J Neuroimaging 18(4): 402-406.
- (1995) Practice parameters for determining brain death in adults (summary statement). The Quality Standards Subcommittee of the American Academy of Neurology. Neurology 45(5):1012-1014.
- Llompart Pou JA, Abadal JM, Güenther A, Rincon JPM, Homar J et al. (2013) Transcranial Sonography and Cerebral Circulatory Arrest in Adults: A Comprehensive Review. ISRN Critical Care Article ID 167468, 6 pages.
- Monteiro LM, Bollen CW, van Huffelen AC, Ackerstaff RGA, et al. (2006) Transcranial Doppler ultrasonography to confirm brain death: a metaanalysis Intensive Care Med 32(12): 1937-1944.
- Ducrocq X, Hassler W, Moritake K, Newell DW, Shiogai T, et al. (1998) Consensus opinion on diagnosis of cerebral circulatory arrest using Doppler sonography: Task Force Group on cerebral death of the Neurosonology Research Group of the World Federation of Neurology. J Neurol Sci 159(2): 145-150.
- Ducrocq X, Braun M, Debouverie M, Junges C, Hummer M, et al. (1998) Brain death and transcranial Doppler: experience in 130 cases of brain dead patients. J Neurol Sci 160(1): 41-46.
- Reinhard M, Roth M, Muller T, Czosnyka M, Timmer J, et al. (2003) Cerebral Auto regulation in Carotid Artery Occlusive Disease Assessed From Spontaneous Blood Pressure Fluctuations by the Correlation Coefficient Index. Stroke 34(9): 2138-2144.
- Aries MJ, Elting JW, Keyser JD, Kremer BP, Vroomen PC (2010) Cerebral Auto regulation in Stroke a review of Transcranial Doppler studies. Stroke 41(11): 2697-2704.
- Panerai RB (2009) Transcranial Doppler for evaluation of cerebral Auto regulation. Clin Auton Res 19(4):197-211.
- Schmidt B, Reinhard M, Lezaic V, McLeod DD, Weinhold M (2016) Auto regulation monitoring and outcome prediction in neurocritical care patients: Does one index fit all? J Clin Monit Comput 30(3): 367-375.
- Panerai RB (2008) Cerebral auto regulation: From models to clinical applications. Cardiovasc Eng 8(1): 42-59.
- Giller CA (1991) A bedside test for cerebral auto regulation using transcranial Doppler ultrasound. Acta Neurochir (Wien) 108(1-2): 7-14.
- Tiecks FP, Douville C, Byrd S, Lam AM, Newell DW (1996) Evaluation of impaired cerebral auto regulation by the valsalva maneuver. Stroke 27(7): 1177-1182.
- Schondorf R, Stein R, Roberts R, Benoit J, Cupples W (2001) Dynamic cerebral auto regulation is preserved in neurally mediated syncope. J Appl Physiol 91(6): 2493-2502.
- Levine BD, Giller CA, Lane LD, Buckey JC, Blomqvist CG (1994) Cerebral versus systemic hemodynamics during graded orthostatic stress in humans. Circulation 90(1): 298-306.
© 2018 Surjya Prasad Upadhyay,. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






