- Submissions

Full Text
Developments in Anaesthetics and Pain Management
The Historical Perspective of Local Anesthetics
Deepak Bhimana1* and Vaishnavi Bhimana2
1Consultant Oral and Maxillofacial Surgeon, India
2Senior Resident, Department of Pediatrics, India
*Corresponding author: Deepak Bhimana, Consultant Oral and Maxillofacial Surgeon, India
Submission: Dec 18, 2017;Published: May 23, 2018

ISSN 2640-9399 Volume1 Issue3
Abstract
Pain is a phenomenon wisely initiated by nature as a warning sign of a condition that may be detrimental to our bodies. The efforts of humankind to find the means to control pain presents as one of the greatest challenges in medicine. The main aim of this article is to list out the forgotten history and evolution of local anesthesia. The comfortable use of today’s local anesthetics was a gift from the past, where like in all areas of studies is a process of many trial and errors perfecting the techniques and compounds used.
Keyword: Local anesthesia; Pediatric anesthesia; History of anesthesia; Erythromycin
Abbreviations: CNS: Central Nervous System; NE: norepinephrine; DA: Dopamine; PABA: Para-Amino Benzoic acid
Introduction
From the earliest antiquities down through the centuries, humans have resorted to many resorted to many measures including superstitions in an effort to blunt this noxious stimulus, yet the knowledge about human body grew, so did our understanding about pain. As Hippocrates once stated “Divinum est opus sedaredolorem“– divine is the work to subdue pain [1]. Some of the earliest references to the use of pain-reducing compounds were found in Homer’s “Odyssey,” when Helen gave Ulysses and his comrades the “sorrow easing drug,” which consisted of a mixture of poppy and Indian hemp. During the siege of Troy, the Greeks used anodyne and astringents to ease the pain of their wounds, completely unaware of its antiseptic property [2]. In the early times, the Assyrians applied pressure over the carotid to cutoff the blood supply to the brain, thus producing a transient syncopal like episode; to obtain a certain degree of anesthesia during circumcisions [3]. This may explain why the literal Greek and Russian translation of carotid artery is the artery of sleep [4]. In the year 50 ad, Pedanius Dioscorides is said to have made the first attempt to produce an anesthetic paste allow in to act as a topical anesthetic. By pulverizing Memphisstone and mixing it with vinegar, the resultant carbonic acid produced a cold stimulus, causing anesthesia over the affected area [5].
It was not until the nineteenth century that the literature began to first describe a chemical with some anesthetic properties. Supposedly, dating back to 1532, the Indians in the highlands of Peru chewed the leaves of coca shrub to relieve fatigue and hunger and to produce a feeling of exhilaration [6-8]. Carl Scherzer in 1856 reported the anesthetic properties of the coca leaf. In 1859 a German chemist, Albert Neimann, was given credit for being the first to extract cocaine in its pure form. German chemist by the name of Friedrich Godeke had also isolated the active ingredient, but had called it erythromycin. It was not until 1865 that one of Niemen’s disciples, Wilhelm Lossen, finally determined the correct formulation of cocaine as C17H21NO4 [9].
In the mid-1860s, as anesthesia began to receive more attention, Sir Benjamin Ward Richardson demonstrated the use of ether spray to anesthetize skin [10]. Around the same time a young Viennese physician, Sigmund Freud, became interested in cocaine’s effect on mood and the psyche. He subsequently administered it to a colleague, Ernst Fleischl Von-Marxow, in an effort to free him from his morphine dependence after a thumb amputation [6-7]. It was at the same time that Carl Koller, then an ophthalmology resident at the University of Vienna, began working with Freudian his physiology lab to perform experiments using cocaine. Koller, who had read that cocaine made the tongue go numb, decided to try it on the conjunctiva. He was successfully able to demonstrate cocaine’s activity on various animal species and even himself [7]. Then in 1884, at the Congress of Ophthalmologists held in Heidelberg, Germany, Koller’s findings were read at the conference, propagating the properties of cocaine [7,11]. The newly discovered properties of this drug were used in every important clinic in the world. Many were thrust into using cocaine, without any regard for its potential side effects, leading to many fatalities. By one account between 1884 and 1891, 200 cases of systemic intoxication and 13 deaths were reported [12]. It was not until Reclus and Schleich’s introduction of infiltrative anesthesia that a drop in fatalities was noted [13]. In 1884 at Roosevelt Hospital in New York, Richard John Hall, and William Stewart Halsted were the first to describe regional block or perineuronal anesthesia [6-11]. Using cocaine they performed what today is referred to as infra orbital and inferior alveolar nerve blocks for dental operations and later perfected many other regional anesthetic techniques [7].
By the1890s, the adverse effects of cocaine had been realized, leading to a more cautious approach in its use. As known today, these side effects include cardiac stimulation, peripheral vasoconstriction, and the excitation of the central nervous system (CNS) along with physical and psychological dependence. The hyper excitation of the cardiovascular system was later found to be due to the blockage of norepinephrine (NE) uptake at the neural terminal end [14]. This stimulatory effect on the cardiovascular system, combined with the Vasoconstrictive effects on the coronary vasculature is now known to cause myocardial infarctions in susceptible individuals [15,16]. The euphoric effects and the abuse potential of the drug are related to its ability to block both dopamine (DA) and NE reuptake at key sites within the brain [7-16].
With advances in compounding and a better understanding of organic chemistry, synthetic derivatives of cocaine were developed to prevent its unfavorable side effects. The first major breakthrough was the synthesis of an ester called procaine (Novocain) by Alfred Einhorn in 1904 [17]. It was not until nearly 40 years later that this development was followed by the synthesis of lidocaine (Xylocaine), the first amide local anesthetic, by Nils Lofgren [18]. In comparison, lidocaine possessed a greater potency, with a more rapid onset and most importantly less allergen city [6-20].
Currently, there are more than a dozen local anesthetics, each with a distinct set of properties and side effects. The need to reduce hospital stays, day surgery is becoming a crucial element of a surgical practice. As a result, the use of local anesthetics has become essential in providing expedient service and care to the patient. This trend has been seen in most surgical subspecialties, including oral and maxillofacial surgery. A recent survey of 865 board-certified German plastic surgeons demonstrated an increased use of local anesthetics for cosmetic surgery of the head and neck, with 1% prilocaine as the most popular local anesthetic, followed by 1% lidocaine. Unfortunately, in the same time, adverse cardiovascular reactions are also up by 8.1% [21]. This increased use of local anesthetics, along with the aging population and their associated comorbidities, makes it prudent for clinicians to be well versed in each type of local anesthetic and their distinct properties [22].
Chemistry of Local Anesthetics
Local anesthetics exert most of their clinical actions through the blockage of nerve impulses by inhibiting the normal function of voltage-sensitive Na+ channels. This, in turn, will prevent the noxious stimuli from reaching the brain and producing the sensation of pain. All injectable local anesthetics are composed of three structural domains: aromatic residue, intermediate chain, and amino terminus (Figure 1).
Figure 1: Local Anaesthetic Structure.
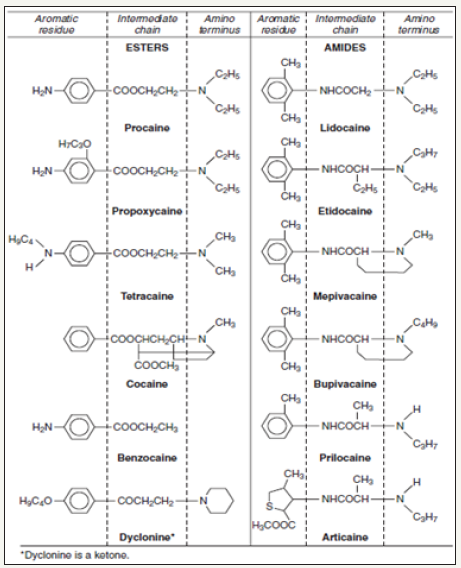
The aromatic or lipophilic portion of the molecule allows the drug to penetrate lipid-rich nerve sheaths and nerve membranes. The intermediate portion of the molecule affords the necessary spatial separation between the lipophilic and hydrophilic portions and divides local anesthetics into two distinct chemical classes: the esters (-COO-) and the amides (-NHCO-). Lastly, the tertiary or second amino end provides the hydrophilic properties of the molecule. This ensures solubility of local anesthetic in the dental cartridge and the interstitial fluid after injection.
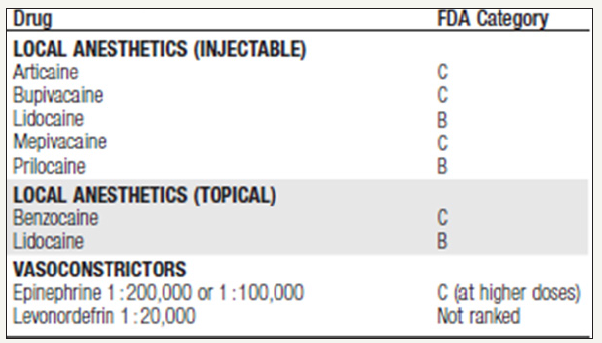
An effortless way to determine whether a local anesthetic is an ester, or an amide is to look at the prefix of the generic name before “-Caine.” If the “I” appears in the prefix, then it is an “I” de, such as lidocaine or bupivacaine. Ester local anesthetics do not contain the letter “I,” such as benzocaine or procaine. The esters, represented by drugs, such as benzocaine, cocaine, procaine, propoxycaine, and tetracaine, are metabolized primarily by plasma pseudo cholinesterase. A byproduct of this metabolism is the formation of Para-amino benzoic acid (PABA), which has been implicated in the development of allergic responses in a small but significant portion of the general population [6-25]. A structurally related chemical, Methylparaben, was used as a preservative in amide local anesthetic solutions until it was discovered that it also produced allergic reactions in susceptible patients [7-26].
Amide local anesthetics are represented by articaine, bupivacaine, lidocaine, mepivacaine, prilocaine, and etidocaine. As a result of their lower risk of allergic reactions, this class of local anesthetics has replaced the esters as the local anesthetic of choice. However, amides, which are metabolized primarily in the liver, can become problematic if they are used in patients with compromised liver function (Figure 2).
Figure 2:
pH effects on LA
The Pharmacodynamics of local anesthetics is affected by several variables, including the pH of the solution and the surrounding soft tissue. Injectable local anesthetics are weak bases with a pKa range of 7.7 to 8.9. This will cause them to exist in two forms: a free base or neutral form and cationic or positively charged form. Because a lipophilic form of local anesthetic is required for better penetration of neuronal tissue, the uncharged free base form readily penetrates neural tissue. Conversely, the cationic form has a more tough time diffusing through the membrane. The Henderson- Hassel Balch equation for weak bases can predict what proportion of local anesthetic will exist in the two ionic states (Figure 3 & 4).
Figure 3: Free Base and Cationic forms of local anesthetic.
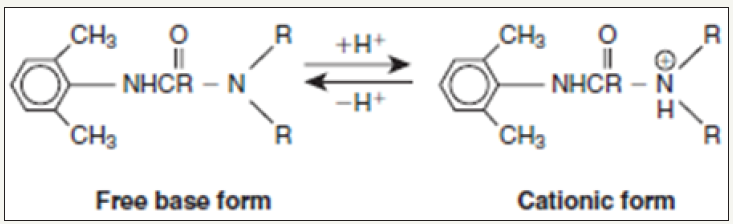
Figure 4: Free Base and Cationic forms of local anesthetic.
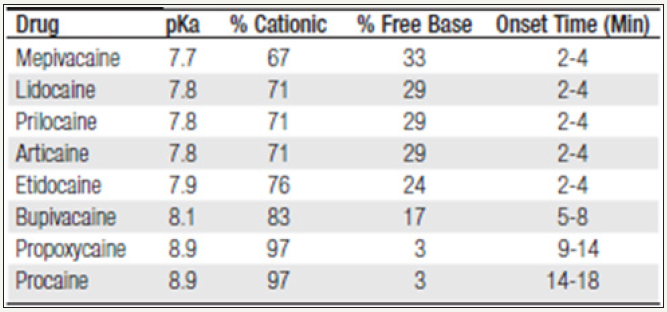
When the local anesthesia is injected into an inflamed or acidic environment, the more hydrophilic portion of the drug will be preponderant, resulting in decreased neuronal penetration and potency. At physiologic pH of 7.4 or higher, local anesthetics with a lower pKa, such as mepivacaine, lidocaine, and prilocaine, will have a higher percentage of their free base available for neuronal diffusion. Studies show that in addition to pH there are other local mediators of inflammation, such as prostaglandins and bradykinin, that can antagonize the effects of local anesthetics [7-27]. Local anesthetics are typically manufactured as an acidic hydrochloride salt; with a pH ranging from 3.5 to 6.0.This improves the water solubility of the local anesthetic and stabilizes the vasoconstrictor. Because of the low pH, approximately 99% of local anesthetic will carry a positive charge. As a result of the buffering capacity of the soft tissue, it is only after injection that local anesthetic is converted into the free base form, capable of penetrating the nerve sheaths.
Mechanism of Action of Local Anesthetics
Local anesthetics can be used in three modes: Topical application, local infiltration, and nerve blockade. All will lead to the blockage of the sensation of pain; touch, temperature, and Proprioception by interfering with the propagation of impulses along peripheral nerve fibers [28]. This is accomplished by are duction in the rate rise in the depolarization phase, leading to a slowing of the action potential. At the molecular level, this deterioration in the action potential is accomplished by blocking the influx of sodium through the excitable nerve membranes [29]. By completely abolishing the inward movement of depolarizing sodium while having little effect on the outward movement of repolarizing potassium, the action potential is blocked [30,31]. Additionally, based on experiments on isolated nerve preparations, there is a direct relationship between the concentration of sodium in surrounding tissue and concentration of local anesthetic needed to completely block the action potential [32]. As the gradient for the influx of sodium becomes more favorable, more local anesthetic is needed to block the action potential.
At sodium channels, both the basic and cationic species appear to be active, with the cationic species entering the sodium channels when they are in the open state and the free base from entering the sodium channels when they are open or closed. Thus, the sequence of events induced by local anesthetic molecules is as follows:
- A reduction in the permeability of the nerve cell membrane to sodium ions,
- A decreased rate of rising in the depolarization phase of the action potential, and
- Failure of a propagated action potential.
Efficacy, Duration of action of LA: [33-36]
There are several factors that influence the efficacy and the duration of local anesthetic action.
- First, and the most obvious, is the accurate anatomic placement of the local anesthetic in the vicinity of the desired nerve.
- The second factor has to do with the actual chemical makeup of the local anesthetic. Properties, such as the solubility of the local anesthetic into the nerve sheath or the presence or absence of a vasoconstrictor, along with the intrinsic activity of the local anesthetic, can make a difference in the efficacy and duration of action (Figure 5).
Figure 5:

Use of Vasoconstrictors with Local Anesthetics
Vasoconstrictors used in anesthetic solutions are epinephrine, norepinephrine and Levonordefrin. In general, vasoconstrictors are classified as adrenergic or sympathomimetic because they are, or closely resemble, the natural mediators of the sympathetic nervous system [37]. Chemically, they are classified as catecholamines because of their direct action on the adrenergic receptors [38,39]. By design vasoconstrictor concentrations within local anesthetics are titrated to approximate the same α-adrenergic activity.
Potential Benefits of Vasoconstrictors [40-43]
- The addition of vasoconstrictors to local anesthetics arose from the desire to reduce or prevent the redistribution of the local anesthetics away from the site of injection.
- use of vasoconstrictors decreases the clearance of the local anesthetic,
- reduces the total required amount,
- increases the duration and depth of anesthesia,
- aids in homeostasis of the surgical wound,
- Presence of a vasoconstrictor there is a delay and a reduction in the peak blood levels of the local anesthetic this is of importance because excessive blood levels of local anesthetics are known to cause systemic toxicity, especially in the pediatric dental population [7-20].
The concentration of vasoconstrictors needs to be somewhere between 1: 200,000 and 1: 100,000 to improve the duration of action. Increasing the concentration of any vasoconstrictor to 1: 50,000 does not only cause significant cardiovascular changes but has also not shown to be of any benefit in duration of action [44].
Potential Systemic Side Effects of Vasoconstrictors
In a typical third molar extraction case, it is not unusual to administer eight cartridges of 2% lidocaine plus 1: 100,000epinephrine. That equates to 288 mg of local anesthetic plus 144 μg of epinephrine. In one study, this amounted to a twentyseven- fold increase in systemic epinephrine levels, resulting in an approximate elevation in systolic blood pressure of about 20 mm Hg, an increase in the heart rate of about 20 beats per minute, and a 52% increase in myocardial oxygen consumption. This may explain why most fatal outcomes have been in older patients with the significant cardiovascular disease. If vasoconstrictors cannot be used, 2% to 3% mepivacaine or 4% prilocaine are the two dental local anesthetics that can provide acceptable plural anesthesia without the use of vasoconstrictors [45, 46] (Figure 6-8).
Figure 6:

Figure 7:
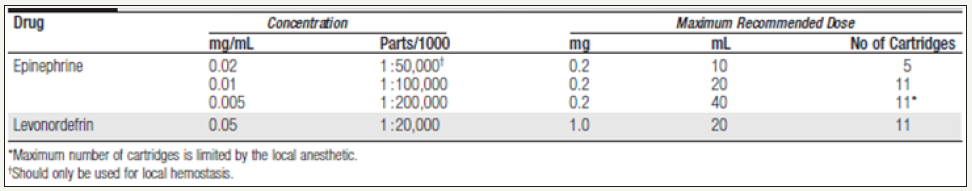
Figure 8:
Localized Complications from Local Anesthesia
Prolonged Sensory Alteration: Prolonged sensory alteration is defined as persistent anesthesia or altered sensation, such as itching or tingling that persists well beyond the expected duration of anesthesia. It can also mean dysesthesia, painful sensation, or hyperesthesia, an increased sensitivity to stimuli. The most common complaints as a result of Paresthesias are speech changes and loss of taste and drooling, with the lingual nerve being the most common cause. Most of these are transient sensory alterations that resolve within 8 weeks, but may also become irreversible [48]. If the damage to the nerve is more severe, the risk of permanent paresthesia is increased.
Hematoma: This is typically seen right after the injection as a localized mass of extravasated blood that follows inadvertent injury to the underlying blood vessels. Vessels most commonly involved are pterygoid plexus of veins, the posterior superior vessels, and the mental vessels. Prevention is the best measure, by reducing the number of times the mucosa is penetrated. If a visible hematoma develops, apply direct pressure. If sub acute, wait 6 hours before the application of ice. Analgesics may be indicated [49].
Pain on Injection: This is most often due to the speed of local anesthetic delivery. If the solution is injected too quickly, it distends the tissue rapidly causing a painful stimulus. Furthermore, if the temperature of the anesthetic is at the extremes of temperature, more pain will be sensed. According to Malamed each cartridge should be injected over a 1-minute period, and the anesthetics should be stored at room temperature.
Needle Breakage: Although uncommon a sudden unexpected movement from the patient or a bent needle can lead to a breakage of the needle, which most commonly occurs at the hub. As expected smaller-diameter needles, such as 30-gauge needles, tend to break more often than larger-bore needles of 25-gauge or higher. To prevent this complication, the following are recommended: The needle should not be bent more than once, never insert the needle up to its hub, do not apply excessive force or redirect the needle once within the soft tissue.
Trismus: Trismus is a relatively uncommon complication after local anesthetic injection. It may be due to injury, spasm, or infection within the medial pterygoid muscle, which is typically violated during an inferior alveolar nerve block. This can be prevented by following the basics of a traumatic injection technique with the application of hot, moist towels to the affected side, in conjunction with arrange of motion exercises, improvement should be noted in about 2 to3 days.
Facial Nerve Paralysis: Because facial nerve (CN VII) traverses the parotid capsule, it is not surprising that transient paralysis can occur if the local anesthetic is deposited within the parotid capsule. Again, prevention is the best medicine, paying attention to the direction and depth of the needle.
Systemic Complications from Local Anesthesia[50]
The initial sign of a systemic overdose may be muscle twitching, tremors, shivering, or even tonic-colonic seizures. This excitatory reaction is thought to be due to the Disinhibition of select inhibitory neurons within the limbic system of the CNS. As the toxic blood concentrations continue to raise, drowsiness, lethargy, sedation, and respiratory depression will follow. Eventually, with extremely toxic levels cardiovascular conductivity will be disrupted leading to cardiac arrhythmia and bradycardia. Long-acting local anesthetics bupivacaine and etidocaine are known to possess a greater risk of producing cardio toxic events than do other local anesthetics. The local anesthetics do sing guidelines are typically based on body weight, and because of this, children tend to be at greater risk for toxic reactions. True dose-dependent toxicities to local anesthetics are frequently reported in the pediatric population [51]. Methemoglobinemia is another unique reaction that can be seen with the use of local anesthetics. It typically occurs 1 to 3 hours after the administration of the local anesthetic. These dosages are based on a patient’s body weight, a 14-kg pediatric patient can theoretically tolerate only one-fifth of the local anesthetic given to a 70-kg or greater adult patient. It should be noted that in very obese children, the maximum dose should be calculated using the ideal body weight and not the true body weight. Malignant hyperthermia (MH) is a rare disorder with an autosomal dominant pattern of inheritance, which is a major cause of anesthetic-related morbidity and mortality in otherwise healthy patients. The role of local anesthetics, stress, and epinephrine in inducing this syndrome is also controversial [52]. Hypersensitivity or allergic reaction to local anesthetics are rare, especially with amide local anesthetics In general if a patient has an allergic reaction to a particular agent in one class, they will most likely be okay with agents from the other class. On the rare occasion that a patient is allergic to both classes of local anesthetics, a 0.5% to 1% solution of diphenhydramine (Benadryl) plus 1: 100,000epinephrine can be used as a viable alternative, with an onset of action of about 5 minutes and duration of at least 30 to 50 minutes. As compared with the actual local anesthetics, this mixture tends to cause more pain on injection and is less efficacious. It also carries a risk of tissue necrosis or dermal sloughing at more than recommended concentrations of 2% to 5% [53].
Local Anesthetic Toxicity Treatment & Management
In the patient with suspected local anesthetic toxicity, the initial step is stabilization of potential threats to life. If the signs and symptoms develop during administration of the local anesthetic, stop the injection immediately and prepare to treat the reaction. Ensure adequate oxygenation, whether by face mask or by intubation. Attention to impending airway compromise, significant hypotension, dysrhythmias, and seizures takes precedence. Once other possible etiologies of the patient’s new symptoms have been excluded, management of the specific symptoms can begin. Benzodiazepines are the drugs of choice for seizure control. Propofol can be used to control seizures but has the risk of potentiating cardiovascular toxicity. Refractory seizures may require neuro muscular blockade (e.g. with succinyl choline) [54-59].
In severe reactions, monitor the cardiovascular system and support the patient with intravenous fluids and vasopressors as required. Small bolus doses of epinephrine are preferred. Vasopressin is not recommended. Hypoxemia and metabolic acidosis may potentiate the cardiovascular toxicity of lidocaine and other local anesthetics. Early control of seizures and aggressive airway management to treat hypoxemia and acidosis may prevent cardiac arrest. Use of sodium bicarbonate may be considered to treat severe acidosis. Cardiac arrest due to local anesthetic toxicity is a rare but well recognized complication that may occur in cases of large overdose, especially those involving inadvertent intravascular injection. These patients have a favorable prognosis if circulation can be restored before hypoxemic injury occurs. Aggressive resuscitation is therefore indicated in most cases. Cardiopulmonary bypass has been used effectively to treat cardiac arrest due to local anesthetic toxicity. Increasing evidence suggests that the intravenous (IV) infusion of lipid emulsions can reverse the cardiac and neurologic effects of local-anesthetic toxicity [54-59].
Infrequently, local anesthetics may provoke an allergic or hematologic reaction. Allergic reactions can be treated with diphenhydramine or, for more serious reactions, epinephrine or corticosteroids. Methemoglobinemia should initially be treated symptomatically. Subsequent treatment is guided by blood levels of methemoglobin; methylene blue and hyperbaric oxygen may be required in severe cases. Local ischemic or nerve toxicities may occur, particularly in the extremities with prolonged anesthesia or use of agents containing epinephrine. Suspected nerve damage should prompt neurologic consultation for urgent peripheral nerve studies. If vascular compromise, such as limb ischemia, is suspected, consult a vascular surgeon immediately. Therapy for extravasation (e.g., warm compresses, phentolamine, and nitroglycerin cream) should be initiated for localized vascular toxicity .Patients with persistent or unresolved significant reactions require admission to a monitored bed for observation, further evaluation, and treatment. Patients who are stable and have minor or easily controlled adverse reactions can be discharged and monitored on an outpatient basis [58,59].
Finally, the prevention of local anesthetic toxicity should always be the primary consideration .Although all adverse reactions cannot be anticipated, complications can be minimized by strict adherence to the guidelines of anesthetic dosing, identification of patients at increased risk, and implementation of appropriate anesthetic application techniques to avoid unintentional intravascular injection.
References
- Ture H (2005) The art of alleviating pain in Greek mythology. Neurosurgery 56(1): 178-185.
- Zorab J (2003) History of Anesthesia. Anesthesia 58(9): 935.
- Wilkinson K (2003) Anesthetists and care of the critically ill child 3. Anesthesia 58(8): 804-805.
- Reiling J (2002) Miscellany. JAMA 287(16): 2042.
- Greene R (1988) Institute for Research in History: History of Medicine, Institute for Research in History and the Haworth press, New York, USA.
- Hersh EV, Condouris GA (1987) Local anesthetics: a review of their pharmacology and clinical use. Compendium 8(5): 374-381.
- Jastak JT (1995) Local anesthesia of the oral cavity, Saunders, Philadelphia,
- Covino BG, Vassallo HG (1976) Local anesthetics: mechanisms of action and clinical use. The scientific basis of clinical anesthesia New York Grune & Stratton, USA.
- Buhler A (1994) Zur erforschung des kookanenusses, Ciba Z 8: 3353- 3359.
- Becker HK (1963) Carl Koller and cocaine, Psychoanal Q 32: 309-373.
- Fink BR (1998) neural blockade in clinical anesthesia and management of pain. In Cousins MJ, Bridenbaugh PO, editors: History of neural blockade, ed 3, Philadelphia, Lippincott-Raven.
- Cocaine (1979) Br Med J 1(6169): 971-972.
- Allen C (1918) Local and regional anesthesia, WB Saunders, Philadelphia.
- Macmillan WH (1959) A hypothesis concerning the effect of cocaine on the action of sympathomimetic amines. Br J Pharmacol Chemother 14: 385-391.
- Lathers CM (1988) Cocaine induced seizures, arrhythmias and sudden death. J Clin Pharmacol 28(7): 584-593.
- Katzung BG (1988) Clinical pharmacology ‘88/’89. A Lange clinical manual, Norwalk, Conn, Appleton & Lange, USA.
- Hadda SE (1962) Procaine: Alfred Einhorn’s ideal substitute for cocaine. J Am Dent Assoc 64: 841-845.
- Dobbs EC (1965) A Chronological history of local anesthesia in dentistry. J Oral Ther Pharmacol 21: 546-549.
- Yagiela JA (1991) Management of pain and anxiety in dental practice. In Dionne R Phero JC editors: Local anesthetics, Elsevier, New York, USA.
- Malamed SF (2015) Handbook of local anesthesia. (4th edn.), St Louis, Mosby, USA.
- Koeppe T (2005) Current trends in local anesthesia in cosmetic plastic surgery of the head and neck: results of a German national survey and observations on the use of ropivacaine. Plast Reconstr Surg 115(6):1723- 1730.
- George EN (2004) Re evaluating selection criteria for local anesthesia in day surgery. Br J Plast Surg 57(5): 446-449.
- Yagiela JA, Neidle EA, Dowd FJ (1997) Pharmacology and therapeutics for dentistry. In: Yagiela JA (Ed.), Local anesthetics, (4th edn), St Louis, Mosby, USA.
- Covino BG, Giddon DB (1981) Pharmacology of local anesthetic agents. J Dent Res 60(8):1454-1459.
- Hersh EV (1993) Local anesthetics in dentistry: clinical considerations drug interactions and novel formulations. Compendium 14(8):1020- 1024.
- Luebke NH, Walker JA (1978) Discussion of sensitivity to preservatives in anesthetics. J Am Dent Assoc 97(4): 656-657.
- Meechan J(1998) How to avoid local anesthetic toxicity. Br Dent J184 (7): 334-335.
- Aceves J, Machne X (1963) The action of calcium and of local anesthetics on nerve cells, and their interaction during excitation. J Pharmacol Exp Ther 140:138-148.
- Strichartz G (1976) Molecular mechanisms of nerve block by local anesthetics. Anesthesiology 45(4): 421-441.
- Hille B (1996) Common mode of action of three agents that decrease the transient change in sodium permeability in nerves. Nature 210(5042):1220-1222.
- Strichartz GR (1973) The inhibition of sodium currents in myelinated nerve by quaternary derivatives of lidocaine. J Gen Physiol 62(1): 37-57.
- Condouris GA (1961) A study on the mechanism of action of cocaine on amphibian peripheral nerve. J Pharmacol Exp Ther 131: 243-249.
- Wilson S, Johns P, Fuller PM (1984) The inferior alveolar andmylohyoid nerves: an anatomic study and relationship to local anesthesia of the anterior mandibular teeth. J Am Dent Assoc 108(3): 350-352.
- Pyle MA (1999) Prevalence and implications of accessory retromolarforamina in clinical dentistry. Gen Dent 47(5): 500-503.
- Chapman PJ, Macleod AW (1985) A clinical study of bupivacaine for mandibular anesthesia in oral surgery. Anesth Prog 32(2): 69-72.
- Giovannitti JA, Bennett CR (1983) The effectiveness of 1.5% etidocaineHCl with epinephrine 1:200,000 and 2% lidocaine HCl with epinephrine 1:100,000 in oral surgery: a clinical comparison. Am Dent Assoc 107(4): 616-618.
- Jastak JT, Yagiela JA (1983) Vasoconstrictors and local anesthesia: a review and rationale for use. J Am Dent Assoc 107(4): 623-630.
- Haas DA (2002) An update on local anesthetics in dentistry. J Can Dent Assoc 68(9): 546-551.
- Sisk AL (1993) Vasoconstrictors in local anesthesia for dentistry. Anesth Prog 39(6): 187-193.
- Chernow B (1983) Local dental anesthesia with epinephrine, Minimal effects on the sympathetic nervous system or on hemodynamic variables. Arch Intern Med 143(11): 2141-2143.
- Meyer FU (1987) Haemodynamic changes under emotional stress following a minor surgical procedure under local anesthesia. Int J Oral Maxillofac Surg 16(6): 688-694.
- Niwa H (2001) cardiovascular response to epinephrine containing local anesthesia in patients with cardiovascular disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 92(6): 610-616.
- Tolas AG, Pflug AE, Halter JB (1982) Arterial plasma epinephrine concentrations and hemodynamic responses after dental injection of local anesthetic with epinephrine. J Am Dent Assoc 104(1): 41-43.
- Yagiela JA (1993) Vasoconstrictors: their role in local anesthesia toxicity. J Jpn Dent Soc Anaesthesiol 21: 261-278.
- Sveen K (1979) Effect of the addition of a vasoconstrictor to local anesthetic solution on operative and postoperative bleeding, analgesia and wound healing. Int J Oral Surg 8(4): 301-306.
- Becker DE (1994)0 Drug interactions in dental practice: a summary of facts and controversies. Compendium 15(10): 1228- 1232 passim.
- Haas DA, Pynn BR, Sands TD (2000) Drug use for the pregnant or lactating patient. Gen Dent 48(1): 54-60.
- True RH (2002) Microprocessor controlled local anesthesia versus the conventional syringe technique in hair transplantation. Dermatol Surg 28(6): 463-468.
- Haas DA (1998) Localized complications from local anesthesia. J CalifDent Assoc 26(9): 677-682.
- Clarkson CW, Hondeghem LM (1985) Mechanism for bupivacaine depression of cardiac conduction: fast block of sodium channels during the action potential with slow recovery from block during diastole. Anesthesiology 62(4): 396-405.
- Moore PA, Goodson JM (1985) Risk appraisal of narcotic sedation for children. Anesth Prog 32(4): 129-139.
- Murray C, Sasaki SS, Berg D (1999) Local anesthesia and malignant hyperthermia: review of the literature and recommendations for the dermatologic surgeon. Dermatol Surg 25(8): 626-630.
- Nakai Y (2000) Effectiveness of local anesthesia in pediatric dental practice. J Am Dent Assoc 131(12): 1699-705.
- Soltesz EG, van Pelt F, Byrne JG (2003) Emergent cardiopulmonary bypass for bupivacaine cardio toxicity. J Cardiothoracic Vasc Anesth 17(3): 357-358.
- Leskiw U, Weinberg GL (2009) Lipid resuscitation for local anesthetic toxicity: is it really lifesaving. Curr Opin Anaesthesiol 22(5): 667-671.
- Cave G, Harvey M (2009) Intravenous Lipid Emulsion as Antidote Beyond Local Anesthetic Toxicity: A Systematic Review. Academic Emergency Medicine. 16(9): 815-824.
- Neal JM, Mulroy MF, Weinberg GL (2012) American Society of Regional Anesthesia and Pain Medicine checklist for managing local anesthetic systemic toxicity. Reg Anesth Pain Med 37(1):16-18.
- Litz RJ, Roessel T, Heller A, Stehr SN (2008) Reversal of central nervous system and cardiac toxicity after local anesthetic intoxication by lipid emulsion injection. Anesth Analg. 106(5):1575-1577.
- Kapitanyan R (2017) Local Anesthetic Toxicity Treatment & Management Local Anesthetic Toxicity Treatment & Management: Approach Considerations Treatment of Central Nervous System Toxicity Treatment of Cardiovascular Toxicity.
© 2018 Deepak Bhimana. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






