- Submissions
Full Text
Developments in Anaesthetics & Pain Management
A Conceptual Model of Trigeminal Neuralgia Network and tDCS Pain Reduction Effect
Mohammadreza khodashenas, Farzad Towhidkhah* and Golnaz Baghdadi
Biomedical engineering department, Amirkabir University of Technology Tehran, Iran
*Corresponding author: Farzad Towhidkhah, Biomedical engineering faculty, Amirkabir University of Technology Tehran, Iran
Submission: November 15, 2017; Published: January 09, 2018

ISSN 2640-9399 Volume1 Issue2
Abstract
Trigeminal Neuralgia (TN) is an attacking, abrupt and electric-shock headache in the realm of one or two branches of trigeminal nerves. It is one of the most severe neuropathic pains ever known. By considering main known regions of the brain involved in TN, we made a conceptual model named TN pain neuro matrix. Then we took an external stimulation into account and assayed the different possible approaches about how it may be concluded to pain relief.
Keywords: Conceptual modeling; Pain management; Pain network; Trigeminal neuralgia; Transcranial direct current stimulation
Abbreviations: TN: Trigeminal Neuralgia; tDCS: Transcranial Direct Current Stimulation; S1: Primary Somatosensory Cortex; S2: Secondary Somatosensory Cortex; IC: Insula Cortex; ACC: Anterior Cingulate Cortex; PCC: Posterior Cingulate Cortex; PF: Prefrontal Cortex; PAG: Periaqueductal Gray; M1: Primary Motor Cortex; SMA: Supplementary Motor Area; PB: Parabrachial Nucleus; ISVT: Interstitial Nucleus of the Spinal Trigeminal Tract; DRG: Dorsal Root Ganglion; VPL: Ventral Posterolateral Nucleus; VPM: Ventral Posteromedial Nucleus; RF: Reticular Formation; SCA: Superior Cerebellar Artery; VAS: Visual Analogue Scale; nBR: Nociceptive Blink Reflex; PREP: Pain-Related Evoked Potentials; MRI: Magnetic Resonance Imaging; H-MRS: Proton Magnetic Resonance Spectroscopy; TG: Trigeminal Ganglion; CeA: Central Nucleus of Amygdala; PSN: Principle Sensory Nucleus; STN: Spinal Trigeminal Nucleus.
Introduction
Pain is not a specific clear located dysfunction. Sensory, motor, associative, autonomous, and limbic systems are involved in the processing of pain [1]. Somatosensory (S1, S2, Insula), limbic (Insula, ACC) and associative (PF) structures receive parallel inputs from multiple nociceptive pathways [2]. The sensory-discriminative aspect of pain may consist of the primary somatosensory cortex (S1) [3-7], secondary somatosensory cortex (S2) [4,6] and sensory nuclei of thalamus [3,6,8]. Another aspect of pain is the affective motivational and emotional dimension of pain, which also consists of limbic system (ACC and insula [2,6,9,10]) and diverse structures such as ACC [2,4,6-11], PCC [1], PF [2,8], PAG [2,8], medial nuclei of thalamus [4,6,11,12] beside intralaminar nuclei [8], insula (IC) [6,8-10], amygdale [8,13], PB nucleus and hypothalamus [8].
The TN is one of the most severe neuropathic pain [14]. It causes unbearable pain in the region of eye, forehead, and jaw, in the ear, or mouth and face that is almost on one side of the face [15]. Some studies have shown that in some cases, the reason of pain might be growing the blood vessel called SCA that causes duress and vibration on the trigeminal ganglion near pons (middle part of the brainstem) connection. Therefore, this force cause transmitting some improper sensory signals to thalamus that makes pain happens. Although, the main reason of TN remains idiopathic.
There are several studies about pain and rare about TN. We can divide them into some categories. Some of the studies perform specific experiments to assess the amount of pain in different groups or therapeutic methods. For pain assessment, usually, the VAS, nBR, and PREP are measured, like [16]. In some other studies, pain pathways and different regions that involve in pain processing have been investigated. These studies may utilize MRI or H-MRS. In one study, the gray matter volume reduction has been found using MRI. The results of these studies led to find regions correlated with pain [17]. Some other studies have investigated the pain mechanism in cellular biology [18]. The results of these studies show the pathology and pathogenesis of TN. On the other hand, there are some studies that have suggested some pain processing mechanism [19].
According to the results of previous studies, there are different approaches to TN therapy. The first treatment is using medicines like carbamazepine. Another therapy is surgical interventions like the micro-vascular decompression and stereotactic radiotherapy [20]. Transcranial direct current stimulation (tDCS) is another option that has been recently used for pain relief. It is a non-invasive therapy. In this method, a weak electrical direct current (usually 1 or 2mA) has been applied to stimulate a specific part of the scalp [20,21]. This method has been also used for pain reduction in people who suffer from TN. Stimulation of motor cortex (M1) is more common among other regions of the brain [20,22,23]. Although the positive effect of tDCS on pain relief has been shown, the main neurobiological mechanism of this method has not been completely understood yet. In this study, considering the results of previous studies involved brain regions in TN, we have proposed a conceptual model consisting some well-known regions called TN neuromatrix. Then, according to the model, we have provided some suggestions about the possible effect tDCS.
Methods
Putting the blocks of involved parts of the brain together, we developed a pain neuromatrix diagram. However, it contains the substantial blocks. Because considering the whole parts of the brain that are involved in pain processing is so sophisticated and need much more elaboration.
Pain pathway
There are several parts of the brain that involve in pain processing. As mentioned above, TN begins from the root of the nerve and trigeminal ganglion (TG) that involved in pain processing pathway [12]. On the other hand, somas of face neurons are in TG and somas of other neurons are in DRG region of dorsal spinal. Trigeminal afferents project by means of the trigeminal ganglion to the mesencephalic nucleus, the principal sensory nucleus (PSN), the interstitial nucleus of the spinal trigeminal tract (ISVT), and the spinal trigeminal nucleus (STN) [24]. So if the signals come from the face, they directly go to the brainstem and then project to the brain. Also, Vc near pons is trigeminal brainstem sensory nucleus [7] that receives not only pain signals from trigeminal ganglion (5th cranial nerve), but also 7th, 9th and 10th cranial nerves [25].
Brain stem: After trigeminal ganglion, the nociceptive signals reach to different parts of the brainstem [26,27]. Brainstem consists of many diverse regions participating in pain pathway like reticular formation [8,13]. Brainstem has the role of consciousness and awareness of pain [8],blocking input signals some deal, heart rate and blood pressure and respiration regulation (that may be due to its nuclei connectivity to hypothalamus and amygdala) [8]. Reticular formation has a reciprocal connectivity with amygdala[13]. Medulla in the lower part of the brainstem in the pain pathway [3,7,27-29] that also innervated from PAG [28]. Pons is in the higher region of the brainstem [3,24,27] that receive signals from ISVT [24]. Brainstem also consists of trigeminal nuclei [3,7,12,16,24,30], PB nucleus, and PAG. Brainstem projects signals to different nuclei of thalamus [7,28] especially VPL,VPM regions [3,8,12], and amygdale [13]. Para-brachial nucleus (PB) is between midbrain and pons that has connections to thalamus [8,13], to amygdale [1,2,8,13], and to hypothalamus [2,8,13,28].
Peri-aqueductal gray (PAG): Peri-aqueductal gray or PAG is one of the substantial main parts of the pain-mediating process that is in the middle part of the brainstem. This region has a connection with amygdale [8] that may be reciprocal [13], and also has a reciprocal connection with hypothalamus [8]. It also receives signals from thalamus [31], insula, and hypothalamus [28]. PAG may engender the endogenous opioids for relieving pain [2,5,8,13,19,27-32] e.g. encephalin that is secreted in the brain and spinal cord and acts as a painkiller.
Basal ganglia: Basal ganglia may also involve in the pain network [2,6,12]. It receives information from prefrontal cortex [2] and projects them to thalamus [2,12].
Amygdala: The amygdala is a region in the temporal lobe may be involved in assigning emotional significance to environmental information. It has a role in triggering adapted physiological, behavioral and affective responses [13].The amygdala, especially its central nucleus (CeA), has also participated in the affective and emotional dimension of pain and controlling autonomic activity [8,13]. During persistent pain states (neuropathic, inflammatory, visceral), a long-lasting functional plasticity of amygdala central nucleus activity contributes to an enhancement of the pain experience, including hyperalgesia [13]. Amygdala gets nociceptive information from insula [2,13], secondary somatosensory cortex (S2) [1], prefrontal cortex [13], PB nucleus [1,2,8,13], and anterior cingulate cortex (ACC) [2,28]. It projects to ACC (so it may be are ciprocal connection with ACC), posterior cingulate cortex (PCC) [1] and prefrontal cortex [2,13]. It may also have some reciprocal interactions with thalamus [1,13], hypothalamus [13], reticular formation [13], S2 [13], and PAG [13].
Hypothalamus: Hypothalamus has participation in the affective and emotional dimension of pain and controlling pain responses reactions in particular autonomic activities, like sweating, respiration, heart rate or blood pressure [1,2,8,13,28]. It receives pain information from ACC [1] and PB nucleus [2,8,13,28]. It has reciprocal connections with amygdale [13] and send information to PAG [28] or have a reciprocal connection with PAG [8].
Insula: Insula plays a role in different functions of pain [1-3,5,811,13,17,27-29,31]. It is well situated to participate in cognitive and memory-related aspects of pain perception, considering its direct anatomical connections with the prefrontal cortex too [9] .The anterior IC is part of the limbic system and is sometimes implicated in the perception and processing of affective aspects of pain [9]. Negative emotional states also enhance pain-evoked activity in limbic regions, such as ACC and IC [2]. Insula gets information from thalamus [2,6,8,28] and S2 [2] or may have a reciprocal connection with S2 [1]. Then it projects information to amygdale [2,13], PAG [28] and ACC [1,2].
Thalamus: Another main region that involves in TN network is thalamus. Thalamus is one of the major structures that receive pain signals from diverse pain pathways [1-8,11,13,16,26-31,33,34]. Essential nuclei are the VPL [8,12,30], VPM [3,5,8], anterior nuclei [5,33], medial nuclei [1,8,11,12] and intralaminar nuclei [1,8]. Thalamus is not merely a relay center but is involved in processing nociceptive information before transmitting the information to various parts of the cortex. The thalamic nuclei are involved in both sensory-discriminative and affective-motivational components of pain. Generally, each group of the nucleus has prominent functions in one component. For example, the ventro-basal complex has a role in sensory-discriminative component and intralaminar nuclei are involved in the affective-motivational component that projects to different parts of the limbic system. VPL nucleus precisely encodes the intensity of noxious stimuli. Its NMDA receptors play a major role in hyper-analgesia, thereby, by blocking them; pain reduction may conclude [8]. Another thalamic structure, posterior complex (Po), has a relationship with the insular cortex and probably has an important role in the motivational-affective responses of pain [8]. Thalamic nucleus sub-medius (Sm) has a connection with ventro-lateral orbital cortex (VLO) and PAG that may form a part of descending inhibiting system and modulates nociceptive inputs [8]. The thalamus is also part of a network that modulates nociceptive information [8]. Thalamus process the nociceptive information coming from brainstem [7,28] especially to VPL and VPM regions[3,8,12], PB nucleus [8,13], basal ganglia [2,12], and primary motor cortex (M1) [26,27,30] (especially to VPL[30]), and projects them to different parts of the brain such as insula[2,6,8,28], posterior cingulate cortex[1], S2 [2,4,6], primary somatosensory cortex (S1) [2-4,6,8,28,34], prefrontal cortex (from medial nuclei)[8] , ACC[2,28] especially from medial [1,4,6,8] and intralaminar nuclei[1], and PAG[31]. It has some reciprocal interaction with amygdala [1,13] and also M1 [29] especially VL and anterior nuclei involve in M1 [5].
Anterior cingulate cortex (ACC): ACC has an important role in pain processing [1-12,16,17,27-29,33]. ACC is a limbic region involved in the processing cognitive motivational-affective aspects of pain, thereby pain-evoked activity in it will enhance during negative emotions [2,6,9], thus it may be a useful target for therapies aiming at the pain control [4]. The cingulate cortex also as the main generator of pain-related cortical potentials has been thought to play a major role in cortical plasticity [16]. Associative nuclei in the ACC also control pain responses reactions in motor reflexes and behavior [1]. It receives the information from insula [1,2], amygdale [1] and thalamus [2,28] especially from medial [1,4,6,8] and intralaminar nuclei[1] andprojects them to amygdala [2,28] (so it may be reciprocal), M1[1], supplementary motor area (SMA) [2] and hypothalamus[1]. It has some reciprocal connections with prefrontal cortex [2] and posterior cingulate cortex (PCC) [2]. PCC has a reciprocal interaction with ACC [2], gets information from thalamus [1], amygdale [1] and S2 [2] then projects them to the prefrontal cortex [1].
Supplementary motor area (SMA): Activation of the supplementary motor area (SMA) is consistent with its proposed role in complex cognitive aspects of sensory-motor integration and the recall of motor memories [9]. SMA gets the information from ACC [2] and has a reciprocal interaction with M1 [2].
Prefrontal cortex (PF): Prefrontal cortex (PF) also involves in pain pathway [1,2,5,8,11,13,17,26,29,33,35]. PF has some reciprocal connectivity with M1 [35] and ACC [2]. It receives pain signals from PCC [1], thalamus [8], amygdale [2,13], and M1 [26], and projects information to basal ganglia [2] and amygdale [13].
Secondary somatosensory cortex (S2): The somatosensory cortex (particularly the S2) is responsible for the sensory- discriminative function of pain [2,4]. In sustaining pain, which could lead to plastic changes in the brain and spinal cord, imbalance of GABA and glutamate in S2 area, with a relative decrease in GABA and an increase in glutamate, cause an increase in the local cortical excitability [4]. Secondary somatosensory cortex has an increased activity in pain condition [1,2,4,6,9-11,13,30]. It has some reciprocal connection with S1 [12], amygdale [13] and insula [1]. S2 receives nociceptive signals from S1 [30] and thalamus [2,4,6] and sends the signals to PCC [2], insula [2], amygdale [1] and S1[1].
Primary somatosensory cortex (S1): Primary somatosensory cortex (S1) is one of the main cortical regions in pain or TN neuro matrix [1-10,12,17,28-30,34,35]. S1 contains neurons that code spatial, temporal, and intensive aspects of innocuous and noxious stimuli [6]. Primary somatosensory cortex plays an important role in the discrimination of stimuli [8] that is responsible for the sensory-discriminative experience of pain [4]. S1 also may play a role in the short-term memory of intensity and spatial aspects of noxious stimuli [9]. Therefore, central pain processing, such as the trigeminal somatosensory pathway, S1, and ACC results suggest that repeated attacks of pain (like what we have in TN) may lead to central sensitization [27].It has some reciprocal interaction with M1 [2,30,35] and S2 [2]. S1 receives nociceptive information from S2 [1], and thalamus [2-4,6,8,28,34], and send some information to S2 [30].
Primary motor cortex (M1): Although the primary motor cortex (M1) is not considered regularly as one of the pain neuro matrix, it plays a key role in modulation of pain in different chronic pain syndromes [1,2,5,26,29,30,35]. Modulation of the activity of this cortical area induces significant analgesic effects [29]. It has some reciprocal connection with prefrontal cortex [35], S1 [2,30,35], SMA [2]. It receives direct information from ACC [1] and sends them to prefrontal cortex [26], brainstem [26,27] and thalamus [26,27], and especially VPL[30]. As PAG, ACC, PF, insula, and thalamus are activated when subjects are distracted from pain, so these regions may be involved in the modulatory circuitry related to attention [2,31]. The functional interactions between PAG and the posterior thalamus are likely to be involved in the network of pain modulation [8,31].
Results
As a result of the above description about pain processing parts of the brain, the conceptual model of the pain processing pathways of TN has been demonstrated in (Figure 1). As mentioned, the main and substantial blocks of the pain network in the trigeminal neuralgia processing system consists of different regions of the brain. In fact, we intend to simulate from the initial noxious stimuli of TN to somatosensory cortex area of the brain. For this purpose, we consider a block for each region.
Applying tDCS over M1
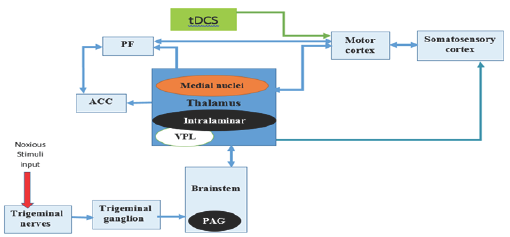
The complex model demonstrated in Figure1 has been summarized in Figure 2. In this figure, the application of tDCS over M1 has been shown. Induced electric current in diverse regions is due to a functional and structural fiber connection between different regions of the brain and M1 that cause alterations in opioid receptors and neurotransmitters (Figure 2).
Figure 1: Pain Neuro matrix specified for TN. TG: trigeminal ganglion, PB nucleus: para-brachial nucleus, PAG: peri-aqueductal gray, BG: basal ganglia, PF: prefrontal cortex, VPL: ventral post-erolateral nucleus, VPM: ventral post-eromedial nucleus, ACC: anterior cingulate cortex, PCC: posterior cingulate cortex, SMA: supplementary motor area, M1: primary motor cortex, S1: primary somatosensory cortex, S2: secondary somatosensory cortex; Colors have no meaning and are merely for better visualization and tracing the pathways in figures.
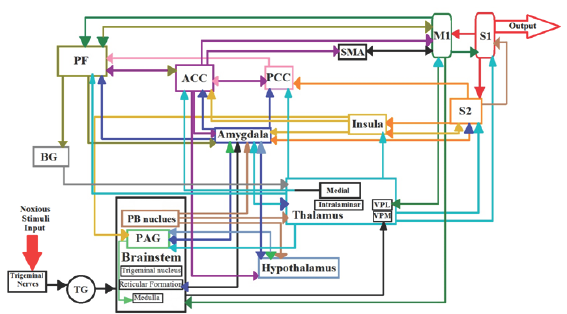
Figure 2: Summarized TN network by applying tDCS over M1.
Discussion
Pain neuromatrix modeling
As a distributed description of each part of the brain and their complex elaborated connectivity, we elicited possible conceptual models that can be used for further studies in the field of the pain. It could be considered not only as a highly detailed model to utilize in pain modulation pathways but also as a good source of computational models that each block can be substituted by a desired computational model. Looking at the TN network, we also get much information about pain modulation interventions. For instance, the diverse location of pain neuro matrix could be considered as our desire intervention (e.g. electrical stimulation).
This conceptual model has been extracted from many studies (including TN or other pains) that contain different experiments on patients. These studies used electroencephalography (EEG), magneto encephalography (MEG), magnetic resonance spectroscopy (MRS), positron emission tomography (PET) and functional MRI (fMRI). Nevertheless, the specific and definite role of every region of the brain remained still uncertain. In our model, we tried to gather all the main results of these studies together.
Effects of tDCS over M1 and pain relief
Descending modulation is important in controlling and inhibiting the transmission of nociceptive stimuli. The PAG, nucleus raphe magnus (in medulla), thalamus and other structures may comprise some part of the inhibitory circuit. Relief of pain mechanism may be due to alteration of opioid receptors and endogenous painkillers such as encephalin and endorphin. Painkillers are associated with inhibition of nociceptive neurons in diverse areas in pain modulation descending pathway. Although M1 may seem unrelated to pain network at first, it has a substantial role in modulating pain e.g. TN.
Stimulation of primary motor cortex will modulate the activity of that region. M1 may enhance its modulatory effect on incoming sensory and nociceptive input by increasing its inhibitory output. As stimulation ofthe peri-ventricular gray (PVG) or the peri-aqueductal gray (PAG) may cause treating chronic pain that engenders endogenous opioids, they may be triggered by M1 stimulation and result in suppression of sensory and affective qualities of the pain. It's not very surprising to claim that by increasing in M1 activity, a decrease in pain will happen (as reported in many studies).
It seems applyingtDCS to primary motor cortex, causes the current flow through superficial (cortical) to inner (sub-cortical) structures. Significant electric fields is generated not only in targeted cortical regions that is consist of skin, scalp and cortical areas (like fiber connections from motor cortex to sensory cortex (M1 to S1 and from S1 to S2 and M1 cortex) but also in deeper areas like insula, thalamus, cingulate cortex, and brainstem regions. Thereby, appropriate stimulation of motor cortex that induces a modulating effect on the sensory cortex, thalamus, may result in treating severe trigeminal neuropathic pain syndromes.
If we want to aggregate our knowledge, it seems that tDCS will affect directly and indirectly to pain relief. The direct effect happens when considering the electric field of tDCS merely on M1. As M1 has fiber and functional connections to diverse regions of the brain, this electric field may affect inhibitory outputs of M1. It seems the modulation happens in GABA as a main inhibitory and Glutamate as a main excitatory neurotransmitter. So the electric field that affects M1 and its connectivity may alter the receptors e.g. NMDA and AMPA, and neurotransmitter e.g. GABA and glutamate, concentration in involved synapses connections to S1, S2, thalamus, brainstem, ACC, PF and other structures. Another effect of tDCS may be the alteration of rest potential of the stimulated area which may cause the modulation of firing and spiking activity of that structure e.g. M1. Ultimately this resting potential may cause other connected regions potential activity too.
The indirect influence of tDCS on pain relief may be due to the distributed electric field in different areas. As mentioned, the significant electric field may reach in deeper areas like insula, thalamus, cingulate cortex, and brainstem regions. Considering the estimated role of each structure, this electric field will also affect the activity of them. One effective influence may be the alteration of neurotransmitters, for instance, serotonin in nucleus raphe magnus or GABA secreted from them edulla. Another effect may also be the modulation of each region neuronal potential activity that may be related to the dynamic activity of that neuron and that region plus connected areas.
It's crucial to mention that, neuronal modeling and considering neuronal activities of each structure seems to be substantial and essential. The neuronal and systematic modeling is not only important for understanding the pain processing but also necessary to anticipate the activity and pain modulation effects.
Conclusion
Notwithstanding rare experimental data about connectivity and diverse connections in the brain, that are critical for building such types of models, and very little network-level modeling in pain processing, the proposed conceptual model is presented. It will be very useful and valuable for further studies. Moreover, the pain neuro matrix of TN is the first conceptual model which consists of main regions of the brain involved in TN. Considering the external intervention such as current stimulation over M1 can be more specific. A lot of works can be done by applying other intervention to other blocks by knowing the physiological background of the system. This block diagram model is not only substantial due to comfort integrated of brain regions for study, but also each block may be substituted by desire model and even diverse stimulation area. A new approach to pain modeling is the systematic point of view by not considering elaborated and detailed of the model. Despite all benefits of this model, it has the capability of improving and become much closer to reality.
References
- B Bromm (2001) Brain images of pain. News Physiol Sci 16(5): 244-249.
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK (2005) Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9(4): 463-463.
- DaSilva AF, Becerra L, Makris N, Strassman AM, Gonzalez RG, et al. (2002) Somatotopic activation in the human trigeminal pain pathway. J Neurosci 22(18): 8183-8192.
- Patrizi F, Freedman SD, Pascual-Leone A, Fregni F (2006) Novel therapeutic approaches to the treatment of chronic abdominal visceral pain. Scientific World Journal 6: 472-490.
- Vaseghi B, Zoghi M, Jaberzadeh S (2014) Does anodal transcranial direct current stimulation modulate sensory perception and pain? A metaanalysis study. Clin Neurophysiol 125(9): 1847-1858.
- Hofbauer RK, Rainville P, Duncan GH, Bushnell MC (2001) Cortical representation of the sensory dimension of pain. J Neurophysiol 86(1): 402-411.
- Dubin AE, Patapoutian A (2010) Nociceptors: the sensors of the pain pathway. J Clin Invest 120(11): 3760-3772.
- Ab Aziz CB, Ahmad AH (2006) The role of the thalamus in modulating pain. Malays J Med Sci 13(2): 11-18.
- Albanese MC, Duerden EG, Rainville P, Duncan GH (2007) Memory traces of pain in human cortex. J Neurosci 27(17): 4612-4620.
- Coghill RC, McHaffie JG, Yen YF (2003) Neural correlates of interindividual differences in the subjective experience of pain. Proc Natl Acad Sci U S A 100(14): 8538-8542.
- Ogino Y, Nemoto H, Inui K, Saito S, Kakigi R, et al. (2006) Inner experience of pain: imagination of pain while viewing images showing painful events forms subjective pain representation in human brain. Cereb Cortex 17(5): 1139-1146.
- Wang Y, Li D, Bao F, Ma S, Guo C, et al. (2014) Thalamic metabolic alterations with cognitive dysfunction in idiopathic trigeminal neuralgia: a multi-voxel spectroscopy study. Neuroradiology 56(8): 685-693.
- P. Veinante, I. Yalcin, and M. Barrot (2013) The amygdala between sensation and affect: a role in pain. J Mol Psychiatry 1(1): 9.
- Baron R, Binder A, Wasner G (2010) Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 9(8): 807-819.
- 15. Okeson JP (2005) Bell's orofacial pains: the clinical management of orofacial pain. Quintessence Publishing Company Chicago, Ill, USA, pp. 592.
- Obermann M, Yoon MS, Ese D, Maschke M, Kaube H, et al. (2007) Impaired trigeminal nociceptive processing in patients with trigeminal neuralgia. Neurology 69(9): 835-841.
- Obermann M1, Rodriguez-Raecke R, Naegel S, Holle D, Mueller D, et al. (2013) Gray matter volume reduction reflects chronic pain in trigeminal neuralgia. Neuro image 74: 352-358.
- Love S, Coakham HB (2001) Trigeminal neuralgia: pathology and pathogenesis. Brain 124(Pt 12): 2347-2360.
- Haeri M, Asemani D, Gharibzadeh Sh (2003) Modeling of pain using artificial neural networks. J Theor Biol 220(3): 277-284.
- Hagenacker T, Bude V, Naegel S, Holle D, Katsarava Z, et al. (2014) Patient-conducted anodal transcranial direct current stimulation of the motor cortex alleviates pain in trigeminal neuralgia. J Headache Pain 15: 78.
- Obermann Mark, Bude Vera, Holle Dagney, Naegel Steffen, Hagenacker Tim, et al. (2014) Anodal Transcranial Direct Current Stimulation Alleviates Pain In Trigeminal Neuralgia. J Headache Pain 15(Suppl 1): E21: 58.
- Pinchuk D, Pinchuk O, Sirbiladze K, Shugar O (2013) Clinical effectiveness of primary and secondary headache treatment by transcranial direct current stimulation. Front Neurol 4: 25.
- Hansen N, Obermann M, Poitz F, Holle D, Diener HC, et al. (2011) Modulation of human trigeminal and extracranial nociceptive processing by transcranial direct current stimulation of the motor cortex. Cephalalgia 31(6): 661-670.
- Ellrich J (2002) Trigeminal nociceptive reflexes. Mov Disord 17(2; SUPP): S41-S44.
- Hall JE (2015) Guyton and Hall textbook of medical physiology. Elsevier Health Sciences.
- Holsheimer J, Nguyen JP, Lefaucheur JP, Manola L (2007) Cathodal, anodal or bifocal stimulation of the motor cortex in the management of chronic pain? Springer 57-66.
- Dasilva AF, Mendonca ME, Zaghi S, Lopes M, Dossantos MF, et al. (2012) tDCS-induced analgesia and electrical fields in pain-related neural networks in chronic migraine. Headache 52(8): 1283-1295.
- Prescott SA (2015) Pain processing pathway models. Encyclopedia of Computational Neuroscience 2181-2187.
- Castillo Saavedra L, Mendonca M, Fregni F (2014) Role of the primary motor cortex in the maintenance and treatment of pain in fibromyalgia. Med Hypotheses 83(3): 332-336.
- Ebel H, Rust D, Tronnier V, Boker D, Kunze S (1996) Chronic precentral stimulation in trigeminal neuropathic pain. Acta Neurochir (Wien) 138(11): 1300-1306.
- Valet M, Sprenger T, Boecker H, Willoch F, Rummeny E, et al. (2004) Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain-an fMRI analysis. Pain 109(3): 399-408.
- Kandel ER, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth AJ (2000) Principles of neural science (5th). McGraw-hill New York 4
- Hsieh JC, Meyerson BA, Ingvara M (1999) PET study on central processing of pain in trigeminal neuropathy. Eur J Pain 3(1): 51-65.
- Zhu YJ, Lu TJ (2010) A multi-scale view of skin thermal pain: from nociception to pain sensation. Philos Trans A Math Phys Eng Sci 368(1912): 521-559.
- Vaseghi B, Zoghi M, Jaberzadeh S (2015) How does anodal transcranial direct current stimulation of the pain neuromatrix affect brain excitability and pain perception? A randomised, double-blind, sham- control study. PloS One 10(3): e0118340.
© 2018 Mohammadreza khodashenas, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






