- Submissions
Full Text
Developments in Anaesthetics & Pain Management
Multi-Modal Neuro-Monitoring and Anesthesia during Neurosurgery in a Child with Wolf-Parkinson-White Syndrome: A Case Report
Bassel Zebian1, Pedro Coelho2, Merate-Kristos Place3, Ali Ansaripour4, Ayala Shirazi5, Alison Goodwin3 and Chulananda Goonasekera3*
1Department of Neurosurgery, Kings College Hospital, UK
2CIinica! Neurophysiology, Neurophys Limited, UK
3CIinica! Neurophysiology, Neurophys Limited, UK
4,5School of Medicine, Kings College, UK
*Corresponding author: Chulananda Goonasekera, Consultant Anaesthetist, Department of Anaesthetics, Kings College Hospital NHS Trust, Denmark Hill, London SE5 9RS, UK
Submission: August 15, 2017; Published: December 13, 2017

ISSN 2640-9399 Volume1 Issue1
Abstract
Wolff Parkinson White (WPW) syndrome associates with life-threatening tachy-arrhythmias. We report anesthetic management of a child with WPW syndrome requiring neurosurgery to resect 3 tumourdeposits (in cerebral hemisphere, cerebellum and spinal cord) under the guidance of intraoperative neuro-physiological monitoring (IONM). Our purpose of this case report is to elucidate the anesthetic limitations in facilitating multiple modes of neuro-monitoring whilst minimizing the risk of triggering an arrhythmia in a child with WPW syndrome. We conclude that multiple modes of IONM is safely possible with a tailor made, proportionately adjusted, inhalational and intravenous general anesthetic with particular attention to the cumulative doses of intravenous agents used.
Keywords: Pediatric, Anesthesia, Neuro-navigation, WPW syndrome
Abbreviations: WPW: Wolff Parkinson White Syndrome; IONM: Intra-Operative Neuro-Physiological; EEG : Electro Encephalo Graphy; MEP : Motor Evoked Potential; SSEPs : Somatosensory Evoked Potentials; FR-EMG : Continuous Free Run Electromyo Graphy; CoMEPs : Cortico-bulbar Motor Evoked Potentials; GCS : Glasgow Coma Score; PSVT : Paroxysmal Supra Ventricular Tachycardia; AF : Atrial Fibrillation; TIVA : Total Intra-Venous Anesthesia
Introduction
WPW syndrome is a rare electrophysiological disorder of the heart, associated with life threatening tachy-arrhythmias. The disorder is probably congenital. However, general anesthesia (especially volatile anesthetic agents) can unmask a WPW pattern, possibly an acquired form [1,2]. These patients carry twice the risk of fatal arrhythmia under general anesthesia. The risk is even greater in neurosurgery, especially during tumor manipulation, attributed to activation of neurogenic reflexes [2]. IONM requires modifications in the anesthetic that may further aggravate this risk. Our case report is a reflection of relief, in the context of providing a 12 hour anesthetic tailored to maintain appropriate physiological conditions conducive for meaningful neuro-monitoring and the safeguards needed to avoid catastrophic arrhythmias in a child with WPW undergoing neurosurgery.
Case Presentation
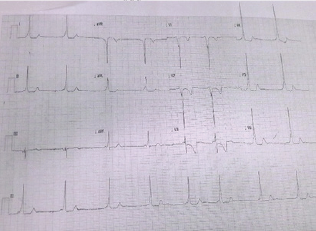
An 11-year-old male of 25kg, known to have WPW syndrome Figure 1, and had a posterior fossa ependymoma resected 2 years earlier was found to have 3 recurrent deposits of the tumor in his cervical spine, frontal lobe and cerebellar area on routine follow- up scanning. Since he was otherwise in considerably good health, a multi-disciplinary decision was made to carry out a precise resection of these secondary tumor deposits, under general anesthesia, facilitated by intra-operative neuro-monitoring. Preoperatively, his GCS was 15/15 and there were no clinical neurological deficits. His WPW syndrome Figure 1 had been stable with no recent symptomatic arrhythmias (Figure 1).
He was induced with intravenous fentanyl 2mcg/kg, propofol 2mg/kg and atracurium 0.6mg/kg following pre-oxygenation and was intubated. Electrodes were placed on multiple sites as described below. He was to be mechanically ventilated throughout the operation, without any further muscle relaxation to enable intraoperative neuro-monitoring. In addition to standard capnography, inhaled oxygen (FiO2) and end tidal anesthetic agent concentration measurement and pulse oximetry, continuous invasive arterial and temperature monitoring was secured, and was catheterized to measure hourly urine output. Remifentanil and propofol infusions were added at variable rates to support minimal use of inhalational agents were required to facilitate neuro-monitoring. There was neither any space nor a need to place a Bi Spectral Index (BIS) monitor as the on-site neurophysiologist was able to gauge the depth of anesthesia via his multi-modal neuro-monitoring.
Figure 1: ECG record showing features of WPW syndrome (decreased PR interval, delta waves, wide QRS and associated ST and T wave changes).
Due to the multi-focal nature of the tumors and their locations, different IONM modalities were applied [3]. The surgeons began with the resection of the frontal lobe tumor, which required the use of EEG with the patient in supine position. Nitrous oxide inhalation (50%) and remifentanil infusions (6.4mcg/kg/hour) supported with minimal concentrations of sevoflurane (0.5 MAC) was used to maintain general anesthesia during cerebral hemisphere surgery. This combination of anesthesia facilitated reliable EEG signals. Once this initial resection was completed, the patient was turned into a prone position and was 'pinned' using a frame. This second phase of surgery involving the posterior fossa and cervical spine required the use of IONM modalities that recorded the functions of the ascending and descending tracts i.e. MEP and SSEP. We agreed that the patient would be unpinned swiftly and turned supine in the event of a catastrophic arrhythmia to enable advanced pediatric life support. The inhalational agent was changed to isoflurane at concentrations below 0.5 MAC supplemented by intravenous propofol (4mg/kg/hr) and remifentanil as above to maintain anesthesia. This combination of agents permitted reliable generation of MEP and SSEP.
During the initial part of the surgery, intravenous propofol infusion was avoided to allow EEG monitoring [4] whilst using minimal concentrations of volatile agents. In the second part of surgery, propofol was administered with extreme restraint to minimize its cumulative dose to prevent triggering of any cardiac events. This promoted SSEPs and MEPs. The operation took 12 hours to complete and was uneventful. The cumulative doses of remifentanil used as an infusion was 2011 mcg (80mcg/kg) and propofol used as an infusion 316.2 mg (12.6mg/kg).
SSEPs were obtained using paired sub dermal needle electrodes applied on the posterior tibial nerve and median nerve bilaterally and scalp. These responses remained stable throughout the operation.
FR-EMG was recorded bilaterally using sub-dermal needles from masseter, orbicularis oculi, orbicularis oris, mentalis, soft palate, vocalis, trapezius and tongue. These showed no clinically significant EMG activity during the procedure.
MEPs were elicited using train of 5-6 pulses from paired sub- dermal needle electrodes placed bilaterally in abductor pollicis brevis and abductor hallucis. A transient reduction of MEP's on left thenar and left abductor hallucis muscles was reported to surgeon during cord manipulation around C2 level. At the completion of resection, MEPs recovered.
CoMEPswere recorded from bilateral masseter, orbicularis oculi, orbicularis oris, mentalis, soft palate, vocalis, trapezius and tongue. CoMEPs remained stable throughout the procedure (Figure 2).
Figure 2: The endotracheal tube wrapped with neuromonitoring electrodes before intubation.

EEG recorded from C3', C4' and Cz' referenced to Fz for the purposes of monitoring anesthetic depth and cortical blood perfusion showed no clinically significant EEG activity under anesthesia.
Discussion
Anesthetic management in WPW syndrome is geared to avoid sympathetic stimulation and myocardial depression. This is to minimize the risk of triggering a PSVT, or AF. In general, being asymptomatic for a long duration lowers the risk (<10%) as in our child. Bolus propofol was the preferred drug of choice for induction as it imposed no effect on the refractory period of the accessory pathway in adults [5]. However, its safety with large cumulative doses was a concern as no safe upper limit has been described.
Anesthetic drugs tend to alter AV node conduction physiology. We chose sevoflurane and isoflurane, as they have no significant effects on conduction of the AV node at the concentrations used. Although sevoflurane was used at the induction phase, this had to be changed to isoflurane especially during MEPs and SSEPs. This is because sevoflurane tends to obtund evoked potentials monitoring even at lower concentrations when compared to isoflurane in our experience. This could be alluded to anesthetic differences in their specific molecular receptors, effects on brain physiology, functional connectivity, brain hemodynamics and metabolism [6,7]. For example, isoflurane induces a 'lower-energy state' anesthetic where brain activity is more limited to local cortical areas [8].
Our goal during the peri-operative period of anesthesia was to avoid any factor that increased sympathetic activity such as pain, anxiety, fear, stress response of intubation/extubation, lighter planes of anesthesia and hypovolemia. This was a challenging task considering the lighter planes of anesthesia needed for IONM modalities without the use of any paralytic agents. It was our impression that the use of neurophysiologist's interpretation of depth of anesthesia via his multi-modal electrodes placed on scalp was more useful than that of a BIS monitor. This is because the BIS index has a large inter-individual variability at different levels of anesthetic depth in children weakening its applicability in pediatric anesthesia [9].
Reliable IONM was essential during this surgery to provide realtime feedback and minimize neuro injury. The use of different IONM modalities limits which anesthetic agents may be administered especially in the pediatric population [10]. Thus, frequent changes were needed between inhalation agents and TIVA to allow both monitoring modalities to be functional whilst keeping the risks minimal. The use of EEG during the first part of the operation required deviation from the normal anesthetic protocols as both TIVA and standard concentrations of inhalational agents interfered with recordings [4].Therefore, we maintained general anesthesia during EEG monitoring using low levels of volatile anesthetics (nitrous oxide and sevoflurane) combined with remifentanil.
The second part of the operation required the use of MEPs and SSEPs. Thus, TIVA was the most appropriate option to maintain adequate depth of anesthesia. Although propofol was safe for induction in WPW syndrome, its prolonged use has reported adverse effects [11] such as propofol infusion syndrome leading to acute refractory bradycardia and asystole, especially in children [12]. Interestingly, there is no toxic propofol level in blood described in literature but a post-mortem blood level of more than 0.02mcg/ ml has been found in a series of reported deaths due to propofol. In addition, the mechanism of the cardiac effects of propofol remains poorly understood. It appears to involve sodium, calcium and potassium ion channels, and the autonomic nervous system and cardiac gap junctions [13].Conservative use of propofol was important in this child with anticipated long duration of surgery. We used a background infusion of 3mg/kg/hr and maintained BIS between 40-60 (as interpreted by neurophysiologist) throughout surgery by titrating the isoflurane concentration to a maximum of 0.5 MAC. This combination of agents allowed us to minimize the cumulative dose of propofol and provided suitable conditions appropriate for complex neurosurgery and reproducible IONM. Isoflurane concentrations below 0.5 MAC served us well in this context. The use of total intravenous anesthesia protocols as in adults would have required substantially larger doses of propofol that may have been detrimental in WPW syndrome. Propofol displays both pro- and anti-arrhythmic effects in a concentration- dependent manner [13]. However, short-term (30 minutes) use of isoflurane 0.8-1.2% or propofol 2mg/kg bolus followed by infusion of 150 micrograms/kg/min has not been shown to alter sinus node recovery time or cardiac conduction in children undergoing radiofrequency catheter ablation for tachydysrhythmias [14].
Stable environmental and physiological factors such as, body temperature, blood pressure and haemoglobin concentration, are also important for reproducible and reliable neuro-monitoring. In addition, constant communication between the surgeon, anesthetist and the neurophysiologist was essential to achieve the best monitoring benefits and outcome whilst avoiding adverse events.
Despite all precautions, PSVT or AF can arise in the perioperative period in WPW. The correct management is to treat using beta-blockers to control sympathetic activation, which slows the anterograde refractory period of the accessory bundle of WPW relative to the AV node, and slows normal cardiac pathways reducing ventricular rate. Early detection of these arrhythmias is considered the key in promoting a successful outcome. In the event of PSVT occurring, vagal manoeuvres should first be initiated, and if haemodynamically stable, lignocaine, adenosine, class 1 anti- arrhythmic drugs or beta-blockers can be used. If haemodynamically unstable, direct cardio-version is needed.
Interestingly, during this case, we noted a lag period of approximately 30 minutes after stopping Sevoflurane for the MEPs to become fully evident and traceable. This 'lag' may be explained by the slow clearance of the inhalational agent dissolved in nerve tissue hindering the nerve conduction.
We had a neurophysiologist on-site right throughout this procedure to consider ambiguity in diagnostics. Although, intervention-mediated recovery from adversely changed MEPs and SSEPs may provide evidence for improved outcomes during IONM, these reversible signal changes may be ambiguous. Therefore, interpretation of these reversible signal changes with the assistance of a neurophysiologist real-time readily permitted and enhanced the value of IONM in prediction and prevention of neurologic deficits. Furthermore, unlike adults, the developing nervous system in pediatric population has peculiar maturation related characteristics in afferent and efferent pathways within the brain and the spinal cord requiring stimulation adjustments tailored for use in children [15].
This case report demonstrates that children with WPW syndrome can be managed uneventfully under general anesthesia, even with extra complexities of managing the multi-focal nature of brain tumors and the use of IONM. We emphasize the importance of thorough preoperative evaluation, and a specifically tailored anesthetic and meticulous intra-operative monitoring. In addition, minimizing triggering factors such as electrolyte imbalance, hypercarbia, hypotension, hypothermia and drug induced myocardial depression is prudent to facilitate a sinus rhythm during a prolonged general anesthetic in a child with WPW.
Acknowledgement
The parents of this child have consented submission of this case report to the journal.
References
- Chhabra A, Trikha A, Sharma N (2003) Unmasking of benign Wolff- Parkinson-White pattern under general anesthesia. Indian J. Anaesth 47(3): 208-211.
- Sharpe MD, Dobkowski WB, Murkin JM, Klein G, Guiraudon G, et al. (1994) The electro-physiologic effects of volatile anesthetics and sufentanil on the normal atrioventricular conduction system and accessory pathways in Wolff-Parkinson-White syndrome. Anesthesiology 80(1): 63-70.
- Sala F, Coppola A, Tramontano V, Babini M, Pinna G (2015) Intraoperative neuro-physiological monitoring for the resection of brain tumors in pediatric patients. J Neurosurg Sci 59(4): 373-382.
- Soriano SG, Eldredge EA, Wang FK, Kull L, Madsen JR, et al. (2000) The effect of propofol on intra-operative electrocorticography and cortical stimulation during awake craniotomies in children. Paediatr Anaesth 10(1): 29-34.
- Warpechowski P, Lima GG, Medeiros CM, Santos AT, Kruse M, et al. (2006) Randomized study of propofol effect on electrophysiological properties of the atrioventricular node in patients with nodal reentrant tachycardia. Pacing Clin Electrophysiol 29(12): 1375-1382.
- Uhrig L, Dehaene S, Jarraya B (2014) Cerebral mechanisms of general anesthesia. Ann Fr Anesth Reanim 33(2): 72-82.
- Hudetz AG (2012) General anesthesia and human brain connectivity. Brain Connect 2(6): 291-302.
- Williams KA, Magnuson M, Majeed W, LaConte SM, Peltier SJ, et al. (2010) Comparison of alpha-chloralose, medetomidine and isoflurane anesthesia for functional connectivity mapping in the rat. Magn Reson Imaging 28(7): 995-1003.
- Rodriguez RA, Hall LE, Duggan S, Splinter WM (2004) The bispectral index does not correlate with clinical signs of inhalational anesthesia during sevoflurane induction and arousal in children. Can J Anaesth 51(5): 472-480.
- Sala F, Coppola, Tramontano V (2015) Intraoperative neurophysiology in posterior fossa tumor surgery in children. Childs Nerv Syst 31(10): 1791-1806.
- Warpechowski P, dos Santos AT, Pereira PJ, de Lima GG (2010) Effects of propofol on the cardiac conduction system. Rev Bras Anestesiol 60(4): 438-444.
- Bray RJ (1998) Propofol infusion syndrome in children. Paediatr Anaesth 8(6): 491-499.
- Liu Q, Kong AL, Chen R, Qian C, Liu SW et al. (2011) Propofol and arrhythmias: two sides of the coin. Acta Pharmacol Sin 32(6): 817-823.
- Lavoie J, Walsh EP, Burrows FA, Laussen P, Lulu JA, et al. (1995) Effects of propofol or isoflurane anesthesia on cardiac conduction in children undergoing radiofrequency catheter ablation for tachydysrhythmias. Anesthesiology 82(4): 884-887.
- Coppola A, Tramontano V, Basaldella F, Arcaro C, Squintani G, et al. (2016) Intra-operative neurophysiological mapping and monitoring during brain tumor surgery in children: an update. Childs Nerv Syst 32(10): 1849-1859.
© 2017 Bassel Zebian, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






