- Submissions

Full Text
Clinical Research in Animal Science
Histological and Histochemical Studies on the Paraepiglottic Tonsil of Goats
Sanju Singla and Pawan Kumar*
Department of Veterinary Anatomy, India
*Corresponding author:Pawan Kumar, Department of Veterinary Anatomy, College of Veterinary Sciences, Luvas, Hisar-125 004, Haryana, India
Submission: September 27, 2023;Published: November 14, 2023

ISSN: 2770-6729Volume 3 - Issue 2
Abstract
The present study aimed to investigate the histology and histochemistry of the paraepiglottic tonsils in goats. Six goats of a local mixed breed, aged 8-10 months and of either sex, were included in the study. The paraepiglottic tonsils exhibited a modified epithelium, characterized by a reticular pattern without distinct strata due to extensive lymphoid tissue infiltration. The propria-submucosa consisted of loose, irregular connective tissue and featured abundant lymphoid follicles and mucous glandular acini. The lymphoid follicles, parafollicular, and interfollicular areas contained small, medium, and large-sized lymphocytes, along with a few plasma cells and macrophages. Histochemical analysis revealed positive reactions for glycogen, mucin, acidic mucopolysaccharides, weakly sulfated acidic mucopolysaccharides, sialomucins, hyaluronic acid, and proteins in the mucous glands. These findings provide important insights into the structural and chemical composition of the paraepiglottic tonsils in goats, contributing to our understanding of their function in the local immune system.
Keywords:Paraepiglottic tonsils; Goats; Histology; Histochemistry; Reticular epithelium; Lymphoid tissue; Mucous glands; Immune system
Introduction
Tonsils are integral components of the mucosa-associated lymphoid tissue, strategically located in areas where the likelihood of infections entering the body is highest. In different species, the oro-pharyngeal and nasopharyngeal regions contain various types of tonsils, including lingual, palatine, tonsils of the soft palate, nasopharyngeal, and tubal tonsils. Additionally, small nodular aggregations at the base of the epiglottis form paraepiglottic tonsils. The epithelia of these tonsils undergo modifications such as Follicle-Associated Epithelium (FAE), Lymphoepithelium (LE), or Reticular Epithelium (RE) at locations infiltrated by lymphoid tissue. Reticular epithelium provides a surface for specialized M-cells to uptake and transport antigens selectively through endocytosis and exocytosis, facilitating interaction between antigen-presenting cells and lymphoid cells [1,2]. Lymphoid tissue present in the tonsils is responsible for respiratory system immunity and the production of mucus that traps foreign particles and particulate matter entering the body during breathing and feeding. This tissue also stimulates the production of B and T lymphocytes. Migration of these lymphoid cells into the systemic circulation occurs through high-endothelial post-capillary venules [3]. The tonsils play a crucial role in cellular, humoral, and innate immunity in the presence of foreign antigens [4]. The histology of paraepiglottic tonsils has been studied in various species, including cattle [5], sheep [2,6-8], Goats [9], Buffaloes [10], and Pigs [11,12]. However, there is a lack of literature specifically focusing on the histology and histochemistry of paraepiglottic tonsils in goats. Consequently, this study aims to address this research gap by investigating the histological characteristics of paraepiglottic tonsils in goats which is essential for gaining insights into their function and contribution to the goat’s immune system.
Objectives
The study aimed to enhance our knowledge of the paraepiglottic tonsils in goats, offering valuable information for comparative anatomy and immunology studies across different animal species and ultimately contributing to a broader understanding of the immune system in goats.
Materials and Methods
In the present study, paraepiglottic tonsils were collected from six healthy goats aged 8-10 months, irrespective of sex. The goats belonged to the local mixed breed and were obtained from a local slaughterhouse immediately after death. No permission from the Institutional Animal Ethical Committee was required for this study. The collected tissues were fixed in a 10% neutral buffered formalin solution for 48 hours and subsequently processed for routine paraffin microscopy. The paraffin sections of 5μ were selected for staining by routine Harris hematoxylin and eosin stain, Weigert’s method for elastic fibres, Gomori’s method for reticular fibres [13], Crossman’s trichrome method for collagen fibres [14], Bielschowsky’s method for the axis cylinder and dendrites, Holmes’ method for nerve cells and fibres, McManus’ method for glycogen, Alcian blue method (pH 2.5) for mucosubstances, PAS Alcian blue method (pH 2.5) for neutral and acidic mucopolysaccharides, Mayer’s mucicarmine method for mucin, colloidal iron method for acid mucopolysaccharides [13], mercury bromphenol blue method for proteins, performic acid Alcian blue method for proteins [15], and Ayoub-Shklar method for keratin and prekeratin [13].
Result and Discussion
The paraepiglottic tonsils were observed as small nodular elevations on either side of the base of the epiglottis, as reported in sheep [6,16,17]. However, the presence of paraepiglottic tonsils in goats has been reported to be inconsistent. In pigs, the paraepiglottic tonsils were oval-shaped, elongated masses with a central pitted part and varying numbers of fossules [11,18,19].
The paraepiglottic tonsils were lined by stratified squamous non-keratinized epithelium (Figure 1A & 1B) with different numbers of rows in various strata, including the stratum basale, stratum spinosum, and stratum superficiale, as reported in sheep [2,6,] and pigs [12]. The stratum basale consisted of a single row of epithelial cells resting on the basement membrane. Their oval to round nuclei exhibited fine chromatin material throughout the nucleoplasm, and the nuclei generally contained 1-2 centric or eccentric nucleoli. The stratum spinosum comprised 10-15 rows of nuclei of different shapes and sizes. The cytoplasm of these cells appeared finely granular and eosinophilic, and the presence of small intercellular spaces gave the strata a spiny appearance. The nuclei in the more superficial layers were horizontally oriented with tapering ends. The stratum superficiale consisted of 6-8 rows of horizontally arranged oval to elongated nuclei, exhibiting a fine dusting of chromatin material except for a few clumps. Some of the smaller nuclei in the most superficial layers showed dense basophilic staining due to pyknotic changes. All cell types exhibited finely granular and eosinophilic cytoplasm. Lymphoid cells, mainly lymphocytes, infiltrated between the epithelial cells.
Figure 1:A. Photomicrograph of paraepiglottic tonsils showing stratified squamous non-keratinized epithelium (E) and lymphoid follicle (L). (H&E × 200) Bar 100μ; B. Stratified squamous non-keratinized epithelium (E) modified into reticular epithelium (R) due to infiltration of the lymphoid tissue. (H&E × 200) Bar 100μ; C. Mucous glandular acini (M) and glandular duct (D) in the propria submucosa (H&E x 200) Bar 100μ; D. Stratified squamous nonkeratinized epithelium (E), reticular epithelium (R) and collagen fibres in the propria submucosa (C). (Crossman trichrome × 200) Bar 100μ.
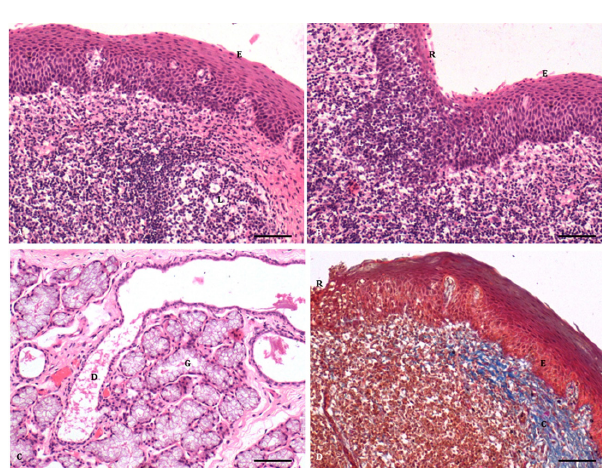
The free surface of the epithelium exhibited unevenness due to folded mucosa, while the deeper portion displayed interpapillary pegs of varying shapes and sizes. In some areas, the stratified squamous non-keratinized epithelium was modified into reticular epithelium without distinct strata due to lymphocyte infiltration (Figure 1B), as reported in Sheep [2,8], Pigs [12], and Buffaloes [10]. Extensive lymphoid infiltration in certain areas made it difficult to discern between the epithelial and lymphoid cells. The reticular epithelium provided a surface for antigen-presenting cells, facilitating the transport and uptake of antigens [20]. It also protected the mucosal surface by producing committed immunocytes [21] and allowed antigen-presenting cells to interact with antigens [20].
The propria-submucosa consisted of loose and irregular connective tissue with abundant collagen fibres, glandular acini clusters, and lymphoid aggregations (Figure 1C & 1D). Fine elastic fibres were isolated in the subepithelial portion but became more concentrated in the deeper portion (Figure 2A). Collagen bundles in the subepithelial portion exhibited a higher concentration near the lymphoid tissue. The lymphoid follicles lacked a typical corona but contained a germinal center composed of small, medium, and large lymphocytes, plasma cells and macrophages. The parafollicular and interfollicular areas showed diffuse infiltration of lymphocytes, blood capillaries, and high endothelial venules. The high endothelial venules, lined by high cuboidal endothelial cells, were consistent with findings in goats [9], pigs [12], and sheep [2, 6]. The lymphoid tissue in the paraepiglottic tonsils of pigs was well developed [11].
Figure 2:A. A dense arrangement of elastic fibres (F) in the propria submucosa (H& E x 100) Bar 200μ; B. Epithelium (E) and acidic mucopolysaccharides in glandular acini (G). (PAS Alcian blue × 100) Bar 200μ; C. Epithelium (E) and Alcian blue reaction in mucous glandular acini (G). (Alcian blue method × 100) Bar 200μ; D. Presence of mucin in the mucous glandular acini (G). (Mayer’s mucicarmine method x 100) Bar 200μ.
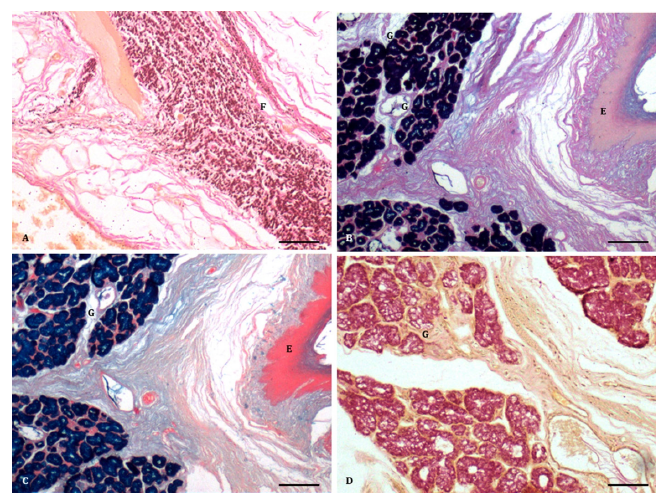
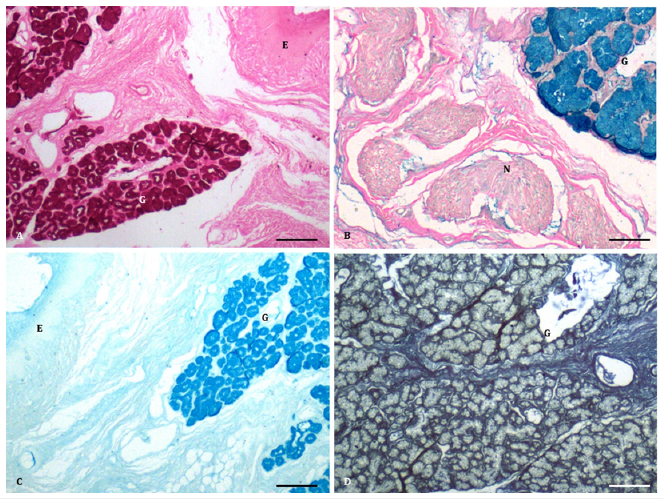
The deeper part of the connective tissue was loose and irregular, containing collagen fibres, nerve bundles, and mucous acini (Figure 2B-2D, Figure 3A-3D). The mucous acini were lined by simple to high cuboidal cells with finely granular and less eosinophilic cytoplasm, giving a vacuolated appearance (Figure 1C). The interand intraglandular ducts were lined by simple to stratified cuboidal epithelium. Additionally, a few small clusters resembling the serous type were found between the mucous acini. In the deepest portion of the propria-submucosa, clusters of serous acini, numerous nerve bundles, ganglia, and collagen fibres were observed (Figure 3B). The glands predominantly contained acidic mucopolysaccharides (Figure 2B) rather than neutral ones, as demonstrated by the PAS Alcian blue method in sheep and buffalo [2,10]. However, in pigs, the secretions strongly tested positive for neutral mucopolysaccharides [12]. The Alcian blue method revealed weakly sulfated acidic mucopolysaccharides, hyaluronic acid, and sialomucins (Figure 2C). Mucous secretions were also positive for mucin, as demonstrated by Mayer’s mucicarmine method (Figure 2D), glycogen (Figure 3A), and acidic mucopolysaccharides by the colloidal iron method (Figure 3B), as reported in pigs [12]. The mucous acini showed a positive reaction for proteins, indicated by the performic acid Alcian blue method, suggesting a concentration of cysteine greater than 4 percent (Figure 3C), similar to buffalo [10]. However, a weak reaction was observed with mercury bromphenol blue (Figure 3D). The intraglandular ducts exhibited positive reactions for different mucopolysaccharides similar to those of the acini. In contrast, the interglandular ducts were negative for both acidic and neutral mucopolysaccharides, except for a few isolated cells positive for mucin.
Figure 3:A. Epithelium (E) and glycogen in the mucous glandular acini (G). (McManus PAS method x 100) Bar 200μ; B. Presence of acidic mucopolysaccharides in the glandular acini (G) and nerve bundles (N). (Colloidal iron method x 100) Bar 200μ; C. Strong reaction for presence of proteins in the mucous glandular acini (G). (Performic acid Alcian blue method x 100) Bar 200μ. C. Weak reaction for proteins (green colour) in the glandular acini (G). (Mercury bromophenol blue x 100) Bar 200μ;
Conclusion
The presence of modified reticular epithelium and its close association with lymphoid tissue are indeed characteristics of the Mucosa-Associated Lymphoid Tissue (MALT). Tonsils, including the paraepiglottic tonsils described in the text, are part of the MALT and play an important role in the local host immune system. The lymphoid tissue within the tonsils contains various immune cells, such as lymphocytes, plasma cells, and macrophages, which are involved in immune responses. The mucous secretions produced by the glandular acini have a function in assisting the immune system. These secretions, which are positive for mucin and mucopolysaccharides, may help in the entrapment and removal of foreign particulate matter, such as pathogens or allergens, present in the respiratory or digestive tract. The mucous layer acts as a physical barrier, trapping potential harmful substances and preventing them from reaching deeper tissues. Additionally, the mucous secretions may contain immune components, such as immunoglobulins, antimicrobial peptides, and enzymes, which contribute to the defense against pathogens. Overall, the presence of MALT in the tonsils and the mucous secretions produced by the glandular acini reflect the important role of these structures in the local immune defense and protection of the respiratory and digestive tracts.
Acknowledgement
The authors are thankful to the Department of Veterinary Pathology for providing the birds of different age groups.
References
- Palmer MV, Thacker CT, Waters W (2009) Histology, immunohistochemistry and ultrastructure of bovine palatine tonsil with special emphasis on reticular epithelium. Veterinary Immunology and Immunopathology 127(3-4): 277-285.
- Kumar P, Singh G, Nagpal SK (2010) Histological studies on the paraepiglottic tonsil of the sheep. Indian Journal of Animal Sciences 80(7): 650-652.
- Kumar P, Timoney JF (2005) Histology and ultrastructure of the equine lingual tonsil. I. Crypt epithelium and associated structures. Anatomia Histologia Embryologia 34(1): 27-33.
- Horter DC, Yoon KJ, Zimmerman JJ (2003) A review of porcine tonsils in immunity and disease. Animal Health Research Review 4(2): 143-155.
- Casteleyn C, Simoens P, Broeck VW (2008) Larynx-Associated Lymphoid Tissue (LALT) in young cattle. Veterinary Immunology and Immunopathology 124(3-4): 394-397.
- Cocquyt G, Baten T, Simoens P, Van Den Broeck W (2005) Anatomical localisation and histology of the ovine tonsils. Veterinary Immunology and Immunopathology 107(1-2): 79-86.
- Sinha MK, Ray S, Gautam AK (2018) Histoarchitectural studies on the organs of upper respiratory tract from nostril to larynx in Garole sheep. International Journal of Current Microbiological Applied Sciences 7: 3362-3368.
- Girgiri IA, Malah MK, Abdulrahman AA (2022) Gross morphological and histological features of larynx in Yankasa sheep (ovis aries). Sahel Journal of Veterinary Science 19(1): 1-9.
- Indu VR, Lucy KM, Ashok N, Maya S (2020) Histomorphological and ultrastructural studies on the paraepiglottic tonsil in goats during postnatal period. Indian Journal of Veterinary Anatomy 32(2): 42-44.
- Girgiri IA, Kumar P (2021) Light and electron microscopic studies on the paraepiglottic tonsil of buffalo (Bubalus bubalis). Journal of Buffalo Science 10: 85-98.
- Liu Z, Yu Q, Li P, Yang Q (2012) Histological and ultrastructural examinations of the porcine tonsils. Anatomical Record 295(4): 686-690.
- Ranjit, Kumar P, Singh G (2015) Histology, histochemistry and scanning electron microscopy of paraepiglottic tonsil of the young pigs. Indian Journal of Veterinary Anatomy 27(2): 30-33.
- Luna LG (1968) Manual of Histologic Staining Methods of Armed Forces Institute of Pathology. (3rd edn), McGraw Hill Book Co, New York, USA.
- Crossman G (1937) A modification of Mallory’s connective tissue stain with a discussion of the principles involved. The Anatomical Record 69(1): 33-38.
- Pearse AGE (1968) Histochemistry: Theoretical and Applied. (3rd edn), Volume 2, Churchill Livingstone, London, UK.
- Girish MH, Jamuna KV, Prasad RV, Manjunatha K, Kumar Bharath ML, et al. (2020) Anatomical localisation of the tonsils in oropharynx and nasopharynx of sheep. International Journal of Chemical Studies 8(2): 1658-1661.
- Telecast C, Cornelissen M, Simoens P, Broeck VW (2010) Ultramicroscopic examination of the ovine tonsillar epithelia. Anatomical Record 293(5): 879-888.
- Casteleyn C, Broeck VW, Simoens P (2007) Histological characteristics and stereological volume assessment of the ovine tonsils. Veterinary Immunology and Immunopathology 20(3-4): 124-135.
- Kostov D, Stefanov I, Tsandev N, Vladova D (2010) Tonsilla paraepiglottica in the Bulgarian White and Landrace pig crosses: morphological traits and some morphometrical investigations. Trakia Journal of Sciences 8(2): 68-72.
- Palmer MV, Stasko J, Ray WT, Thacker TC (2011) Examinations of the reticular epithelium of the bovine pharyngeal tonsil. Anatomical Record 294(11): 1939-1950.
- Brandtzaeg P, Halstensen TS (1992) Immunology and immunopathology of tonsils. Immunology and Immunopathology 47: 64-75.
© 2023 Pawan Kumar. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






