- Submissions

Full Text
Clinical Research in Animal Science
Age Related Histological and Histochemical Studies on the Proventriculus of Broiler Chickens
Asha Mehra and Pawan Kumar*
Department of Veterinary Anatomy, India
*Corresponding author: Pawan Kumar, Department of Veterinary Anatomy, College of Veterinary Sciences, LUVAS, Hisar-125 004, Haryana, India
Submission: May 30, 2023;Published: June 26, 2023

ISSN: 2770-6729Volume 3 - Issue 1
Abstract
The present study was envisaged to explore the histoarchitecture of the proventriculus of broiler chicks at different ages. In every age group, proventriculus had all four tunics i.e., mucosa, submucosa, muscularis and serosa. The luminal surface of the mucosa presented folds of varied heights called plicae, separated by depressions, sulci. The folds had simple columnar epithelium but became cuboidal type towards the bases of the sulci. The cells of these folds showed strong positive activity for the presence of glycogen, acidic mucopolysaccharides, hyaluronic acid, sialomucins, mucins and proteins. The plicae experienced a higher response to various mucopolysaccharides than the bases of the sulci. The lamina propria mucosae had loose irregular connective tissue and showed lymphoid infiltrations in birds of all age groups. Lamina muscularis mucosae was interrupted and present in the form of only a few widely placed smooth muscle fibres. Within the tunica submucosa, the proventricular glands were present. The PAS activity for different mucopolysaccharides and proteins was observed only in primary ducts whereas the secondary and tertiary ducts were devoid of the activity. Tunica muscularis presented an inner thin oblique layer, middle thick circular layer and outermost longitudinal to oblique layer of smooth muscle fibres. Thickness of proventriculus was drastically increased with the advancement of age because of increased glands; however, the thickness of layers of tunica muscularis was not much increased
Keywords:Proventriculus; Simple columnar epithelium; Broilers; Mucopolysaccharides
Introduction
The proventriculus varies in size between species, being relatively small in graminivores but often quite large and distensible in carnivorous birds that ingest large food items [1]. The intermediate zone between the oesophagus and the proventriculus revealed an abrupt change from a stratified squamous to a simple columnar epithelium in red jungle fowl [2]. The proventriculus had an elaborate system of submucosal glands constituting the majority of its wall thickness [3]. It is characterised by mucus-secreting cylindrical cells and several branched glands having oxyntic-peptic cells responsible for secretions of hydrochloric acid and pepsinogen [4]. The mucous over the epithelial surface not only acts as a lubricant but also as a protective barrier to mechanical damage by the ingested food particles. In addition, this may be involved in entrapment and clearance of micro-organisms entered through the oral route. The proventriculus has been studied in different domestic and wild birds ([1,5-7] without any report on age related histological changes including the broiler chickens.
Objectives
The present study was envisaged to correlate developmental histological and histochemical changes of proventriculus at different ages in the broiler chicks which might be of significance in understanding of the physiology of digestion and formulation of feed rations.
Materials and Methods
The present study was conducted on 30 broiler chicks of 7-42 days of age, divided into 6 groups, having 5 birds in each group. The tissues were collected from proventriculus after the post-mortem examination of healthy birds at 7, 14, 21, 28, 35 and 42 days of age. Permission from the Institutional Animal Ethical Committee was not required as the organs were collected only after post-mortem examination at the Department of Veterinary Pathology, LUVAS, Hisar, India. The tissues for histomorphological and histochemical studies were fixed in 10% neutral buffered formalin solution for 48 hours. The tissues were processed for routine paraffin technique and paraffin sections of 5-6μ were cut and stained with following stains: Routine Harris’ hematoxylin and eosin stain for general architecture, Gomori’s method for reticular fibres, Weigert’s method for elastic fibres [8], Crossman’s trichrome stain for collagen fibres [9], Alcian blue method for weakly acidic mucosubstances, hyaluronic acid and sialomucins (pH 2.5), McManus’ method for glycogen (PAS), PAS-Alcian blue method for acidic and neutral mucosubstances, Meyer’s mucicarmine method for mucin, colloidal iron method for acidic mucopolysaccharides [8] and performic acid Alcian blue method [10].
Result and Discussion
The proventriculus of birds was comprised of tunica mucosa, submucosa, muscularis and serosa as documented in most of other avian species, such as Ostrich (Struthio camelus) [11], Guinea fowl (Numida meleagris) [12], Quail [5], Japanese quail [13], Coot bird [6], Falcon [14], and Mallard [1]. The luminal surface of the mucosa represented papillae surrounded by many folds with a concentric arrangement (Figure 1A-D). The number of papillae increased with advancement of age, however, reported absent in the duck [15]. These folds presented the plicae towards the free surface whereas the deeper portion resulted in sulci because of depressions. There was no definite arrangement of these plicae and sulci as in fowl [16] and quails [17]. The papillae lined by simple columnar epithelium (Figure 1-3) showed comparatively more height towards the plicae as compared to the sulci. The nuclei of the columnar cells were basophilic, round to oval, having a linear arrangement towards the base of cells. Some of the nuclei of irregular shapes and larger in size might represent the different functional activity of the cells. The epithelial height was comparatively reduced towards the sulci. The finely granular cytoplasm of all the cells was more eosinophilic toward the plicae than that of sulci. In contrast, a simple cuboidal epithelium was observed in Kadaknath fowl [18], bustards [19], red Jungle Fowl [2], quails [17], and Mallard [1].
Figure 1:Photomicrograph of proventriculus of chick showing simple columnar epithelium (e), proventriculus glands (g), lymphoid infiltration (l) A. at 7 days age (H&E × 40) Bar 500μ; B. at 7 days age (H&E × 100) Bar 200μ; C. at 14 days age (H&E × 100) Bar 200μ; D. at 42 days age (H&E × 40) Bar 500μ.
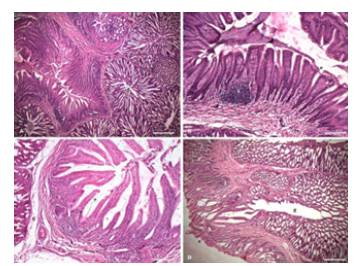
Figure 2:Photomicrograph of proventriculus of chick showing presence of different mucopolysaccharides in the simple columnar epithelium and folds (arrow), and absence in the proventriculus glands (g). A. Glycogen at 7 days age (McManus PAS × 100), Bar 200μ; B. Acidic mucopolysaccharides at 7 days age (PAS AB × 100), Bar 200μ; C. Weakly sulfated mucopolysaccharides, hyaluronic acid and sialomucins at 42 days age (AB × 100), Bar 200μ. D. Mucin at 7 days age (Meyer’s mucicarmine × 100), Bar 200μ.
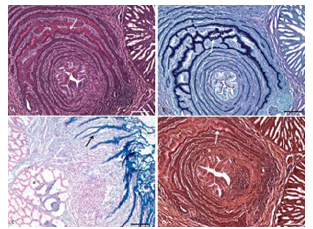
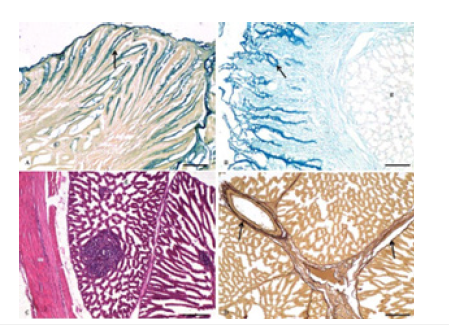
Figure 3:Photomicrograph of proventriculus of chick showing A. Presence of acidic mucopolysaccharides in the simple columnar epithelium and folds (arrow), and absence in the proventriculus glands (g) at 14 days age (Colloidal iron × 100), Bar 200μ; B. Presence of more than 4% content of cysteine in the epithelium and folds (arrow), and absence in the proventriculus glands (g) at 42 days age (Performic acid Alcian blue × 100), Bar 200μ; C. Proventriculus glands (g), lymphoid infiltration (l) and tunica muscularis (m) at 28 days age (H&E ×100) Bar 200μ; D. Presence of elastic fibres (arrow) associated with proventriculus glands and the blood vessels at 14 days age (Weigert’s × 100) Bar 200μ.
These cells were strongly PAS-positive for glycogen, as demonstrated by McManus’ method (Figure 2A). The intensity of the reaction showing the presence of glycogen was more towards the plicae region, as found in red jungle fowl [2]. The columnar cells of plicae showed the predominance of acidic mucopolysaccharides with a very low concentration of neutral mucopolysaccharides (Figure 2B). The positivity was localised mainly towards the apical portion of the cells. The sulci presented only the presence of acidic mucopolysaccharides. The folds, which had a concentric arrangement, showed the presence of only acidic mucopolysaccharides in their cells, with a very low concentration of neutral mucopolysaccharides as reported in mallard [1], blackwinged kite [20], and guinea fowl [12]. However, a positive reaction was seen for neutral mucopolysaccharides, especially in the upper folds and the surface epithelium in duck and pigeon [15]. The presence of neutral and acid mucins aid in protecting the mucosal surface and form a resistant mucosal barrier [20].
The Alcianophilic reaction was comparatively lesser towards plicae, but very strong in the folds and at the base of sulci (Figure 2C) indicating the presence of weakly sulphated mucopolysaccharides, hyaluronic acid, and sialomucins. Mayer’s mucicarmine demonstrated the presence of mucin, and the reaction was again more towards plicae and less towards basis (Figure 2D). The cells along the top of the plicae showed a weaker reaction with the colloidal iron method than those at the base of the sulci, where a very strong reaction was seen, indicating the presence of acidic mucopolysaccharides (Figure 3A). The performic acid- Alcian blue method also demonstrated that the surface epithelium cells lining the surface of plicae had a slightly lesser reaction than those of sulci. The cells of folds presented a very strong positive reaction, indicating the presence of protein, especially more than 4% cysteine content (Figure 3B).
The lamina propria mucosae had loose, irregular connective tissue consisting of collagen, reticular, a few elastic fibres, connective tissue cells, and fine blood capillaries. Isolated lymphoid aggregations observed in the deeper part were also seen in red jungle fowl [2], Kadknath fowl [18], broilers [7] and ducks [21]. The lymphoid cells infiltration has been reported in the surface glandular cells of grey-backed shrike (Lanius tephronotus) and black-tailed crane [22,23]. In contrast, mucous glands with lymphatic infiltration were observed in the lamina propria of ducks and pigeons [15]. A few lymphoid aggregations comprising of lymphocytes of different sizes, plasma cells, and a few macrophages were observed. Some of the lymphoid cells also penetrated into the epithelium. Almost all of the lamina propria was occupied by the lymphoid tissue in the birds of 42 days of age. The lamina muscularis mucosae in the form of widely placed smooth muscle fibres was interrupted. However, it was having two layers in the grey-backed shrike where the inner layer of varying thickness was located upon the simple tubular glands, while the outer layer was a discontinuous longitudinal layer of smooth muscles [22]. The two layers of muscles were separated by the proventricular glands in ducks and pigeons [15]. The muscularis mucosae diverged into two layers at the oesophagus-proventriculus junction in partridge [3].
The tunica submucosa had loose, irregular connective tissue comprising collagen, reticular, and elastic fibres, fine blood capillaries, small to medium-sized blood vessels, and thin-walled structures similar to the venous caverns. In contrast, the submucosa was a thin layer in ducks, pigeons [15], and red jungle fowl [2]. In the deeper part of the submucosa, the proventricular glands were present, which were oriented in the form of lobules of varying shapes and sizes being separated by bundles of collagen, reticular and elastic fibres (Figure 1-3). A similar arrangement was reported in partridge [24], coot birds [6], guinea fowl [12], and mallard [1]. The size of the glands increased with the advancement of age of the birds which might be due to fusion of two or three adjacent lobules of proventricular glands and the formation of large proventricular glands [18] as reported in the present study. These glands did not exhibit any PAS activity or reaction toward the mucopolysaccharides by the methods employed during present study.
Each lobule of the gland opened towards its center where the excretory duct was lined by simple columnar epithelium similar to that of ducks and pigeons [15]. The finely granular cytoplasm of these cells exhibited more eosinophilia towards the supranuclear portion. The lumen of these glands presented varying shapes because of the presence of folds in the excretory duct. Toward the base of the fold of the excretory duct, the epithelium changed to low columnar to high cuboidal cells showing finely granular and comparatively more eosinophilic cytoplasm than those of the excretory duct. Toward the deeper portion, a similar type of secondary duct followed by a tertiary duct was observed. The concentration of the glands drastically increased in 35-42 days old birds. Lobules of the glands arranged in two layers were stacked one upon another. The aggregations of lymphoid tissue started appearing at few places toward the bases of lobules of proventricular glands in birds of 14 days. The lymphoid aggregation associated with secondary and tertiary ducts of the proventricular glands were also observed at 21 and 28 days. The distribution of the lymphoid tissue further increased in birds of 42 days. The histochemical features were almost identical in all the age groups.
The tunica muscularis was constituted by smooth muscles oriented obliquely as outer and inner layers being separated by a thicker middle circular layer (Figure 3C). In contrast to this, an inner longitudinal and an outer circular layer were observed in ducks and pigeons [15], partridges [3], and falcons [14]. However, the longitudinal layer was thicker than the inner circular layer in the case of duck [15], and falcon [14]. Inner thin longitudinal and outer thick circular layers of tunica muscularis were observed in Mallard [1]. The inner circular and outer longitudinal layers were reported in Kadaknath fowl [18]. The outer layer was not uniformly observed; however, at places where it was not observed, it was always the thinnest of the three layers. It was interesting to note that the thickness of proventriculus increased many folds as compared to those of birds of 1st age group because of the increased number of glands but the thickness of layers of tunica muscularis was not much increased. The outermost tunica serosa contained connective tissue fibres, collagen, reticular fibres, and a few elastic fibres. These were also reported in fowl [16], ducks and Pigeons [15], Japanese quail [13], coot birds [6], falcons [14] and black-tailed crake [23].
Conclusion
The folded mucosa of the proventriculus, lined by simple columnar epithelium, was strongly positive for different moieties of carbohydrates. However, the proventriculus glands were devoid of PAS activity for the techniques employed in the present study.
The lymphoid aggregates were observed in the lamina propria, and the submucosa increased with the advancement of the birds’ ages. The histological and histochemical features were almost identical except for the developmental changes in the birds of all age groups.
Acknowledgement
The authors are thankful to the Department of Veterinary Pathology for providing the birds of different age groups.
References
- AI-Saffar FJ, AI-Samawy ERM (2015) Histomorphological and histochemical studies of the mallard (Anas platyrhynchos). Asian Journal of Animal Science 9(6): 280-292.
- Kadhim K, Zuki A, Noordin M, Babjee S (2010) Histomorphology of the stomach, proventriculus and ventriculus of the red jungle fowl. Anatomia Histologia Embryologia 40(3): 226-233.
- Rossi JR, Baraldi-Artoni SM, Oliveira D, Cruz C da, Franzo VS, et al. (2005) Morphology of glandular stomach (Ventriculus glandularis) and muscular stomach (Ventriculus muscularis) of the partrigde Rhynchotus rufescens. Ciecia Rural 35(6): 1319-1324.
- Aksoy A, Cinar K (2009) Distribution and ontogeny of gastrin- and serotonin-immunreactive cells in the proventriculus of developing chick (Gallus gallus domestica). Journal of Veterinary Science 10(1): 9-13.
- Attia HF (2008) Some histological studies on the proventriculus of the quail during pre and post hatching periods. Minufiya Veterinary Journal 5(2): 441-453.
- Batah AL, Selman, HA, Saddam M (2012) Histological study for stomach (proventriculus and gizzard) of coot bird (Fulica atra). Diyala Agricultural Sciences Journal 4(1): 9-16.
- Lambate SB, Mamde CS (2008) Histological studies of proventricular and gizzard glands in broiler. Royal Veterinary Journal of India 4(2): 9-12.
- Luna LG (1968) Manual of Histologic Staining Methods of Armed Forces Institute of Pathology. 3rd (edn), McGraw Hill Book Co., New York, USA.
- Crossman GA (1937) A modification of Mallory’s connective tissue stain with a discussion of principles involved. Anatomical Record 69(1): 33-38.
- Pearse AGE (1968) Histochemistry: Theoretical and Applied. 3rd (edn), Volume I, Churchill, London, UK.
- Cooper RG, Mahroze KM (2004) Anatomy and physiology of the gastro-intestinal tract and growth curves of the ostrich (Struthio camelus). Animal Science Journal 75(6): 491-498.
- Selvan PS, Ushakumary S, Ramesh G (2008) Studies on the histochemistry of the proventriculus and gizzard of post-hatch guinea fowl (Numida meleagris). International Journal of Poultry Science 7(11): 1112-1116.
- Ahmed YAEG, Kmel G, Ahmad, AAEM (2011) Histomorphological studies on the stomach of the Japanese quail. Asian Journal of Poultry Science 5: 56-67.
- Abumandour MM (2013) Morphological studies of the stomach of falcon. Scientific Journal of Veterinary Advances 2(3): 30-40.
- Hassan SA, Moussa EA (2012) Gross and microscopic studies on the stomach of domestic duck (Anas platyrhynchos) and domestic pigeon (Columba livia domestica). Journal of Veterinary Anatomy 5(2): 105-127.
- Hodges RD (1974) The Histology of the Fowl. Academic Press, London, UK.
- Zaher M, Ghareep AWE, Hamdi H, Abu Amed F (2012) Anatomical, histological and histochemical adaptations of the avian alimentary canal to their food habits. Life Science Journal 9(3): 201.
- Das S, Dhote BS, Singh GK, Pandey M (2013) Gross morphometrical and biometrical studies on the proventriculus of Kadaknath fowl. Indian Journal of Veterinary Anatomy 25(2): 74-75.
- Bailey TA, Mensah-Brown EP, Samour JH, Naldo J, Lawence P, et al. (1997) Comparative morphology of the alimentary tract and its glandular derivatives of captive bustards. Journal of Anatomy 191(Pt 3): 387-398.
- Hamdi H, El-Ghareeb AW, Zaher M, Abu Amod F (2013) Anatomical, Histological and histochemical adaptations of the avian alimentary canal to their food habbits: II- Elanus caeruleus. International Journal of Scientific and Engineering Research 4(10): 1355-1364.
- Prasad RV, Kakade K (1990) Histology and histochemistry of proventriculus of domestic duck (Anas platyrhynchos Linnaeus). Mysore Journal of Agricultural-Sciences 25(4): 506-511.
- Zhu L (2015a) Histological study of the oesophagus and stomach in grey-Backed shrike (Lanius tephronotus). International Journal of Morphology 33(2): 459-464.
- Zhu L (2015b) Histological and histochemical study on the stomach (proventriculus and gizzard) of black-tailed crake (Porzana bicolor). Pakistan Journal of Zoology 47(3): 607-616.
- Juliana RR, Silvana MB, Daniela O, Claudineida C, Vanessa SF, et al. (2005) Morphology of glandular stomach (Ventriculus glandularis) and muscular stomach (Ventriculus muscularis) of the partridge Rhynchotus rufescens. Ciência Rural 35(6): 1319-1324.
© 2023 Pawan Kumar. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






