- Submissions

Full Text
Clinical Research in Animal Science
Use of Yeast Culture Residue as A Pre-Harvest Treatment in The Control of Campylobacter in Broiler Chicks
Praise Benson, Hannah Adams, Victor Stanley and Milton Daley*
Cooperative Agricultural Research Center, USA
*Corresponding author:Milton Daley, Cooperative Agricultural Research Center, Texas, USA
Submission: August 12, 2022;Published: September 29, 2022

ISSN: 2770-6729Volume 2 - Issue 3
Abstract
Campylobacter is a primary food pathogen in the food industry that accounts for about 2.5 million human cases annually. It ranks the highest among the 19 bacterial agents and cost about $1.2 billion of the $6.9 billion allotted to annual foodborne illness. Campylobacter can be found in poultry, meat, unpasteurized milk, untreated water, and fish. The two strains of these pathogens C. jejuni (Poultry), C. coli (Humans). In the United States, about 69% of the chickens brought from a local supermarket were contaminated with Campylobacter jejuni. Antibiotics to treat Campylobacter are available, but there is still a need for more research for poultry. It is hypothesized that Yeast culture residue is an antibiotic-based drug alternative that could reduce the cecal population of Campylobacter by blocking its attachment to the epithelial cells. To examine the sensitivity of selected strains of Campylobacter (C. jejuni and C. coli) to the dietary inclusion of yeast culture residue during the pre-harvest stage, two experiments were conducted to examine the use of a Yeast Culture Residue (YCR) as a pre-harvest treatment to control Campylobacter colonization in broiler chicks. Four hundred unvaccinated and unsexed Cornish Rock day-old broiler chicks were separated into four treatment groups: control- uninfected and untreated; Campylobacter infected and untreated; YCR treated and uninfected, and Campylobacter infected and treated with YCR at 2kg/ton of feed. The infected chicks were challenged with a cocktail mixture (10^8 CFU) of ten strains of Campylobacter spp. (5 C. jejuni and 5 C. coli strains). Infected chicks were challenged by crop gavage at 1-day-old. Yeast culture residue was incorporated into the feed, and the chicks were fed from one day of age. In the Campylobacter -free chicks, YCR did not significantly change the volatile fatty acids’ cecal concentration and lactic. Birds infected with the organism had increased concentrations (P< 0.05) of cecal acetic acid (156.1 vs. 125.4), cecal propionic acid (32.50 vs. 24.77), and cecal butyric acid (34.0 vs. 17.1 u mole/gram of cecal contents) in comparison to the control. YCR decreased (P<0.05) cecal lactic acid concentration in Campylobacter and YCR (0.85 and 0.69umole/gram, respectively. A significant reduction in the total number of the Campylobacter (4.22 vs. 6.14Cfu/gram of cecal contents) was observed in the infected birds with the inclusion of YCR when compared with controls. Yeast residue’s dietary inclusion at 2kg/ton could be a useful pre-harvest treatment for reducing Campylobacter colonization in broiler chicks.
Keywords:Campylobacter; Yeast culture residue; Broilers
Introduction
Foodborne illness is currently a global concern, generating much attention to finding solutions to combat the problem. Over the last decade, Campylobacter has emerged as the primary food pathogen in the food industry. Campylobacteriosis accounts for nearly half of the estimated five million human cases annually, ranking the highest among the 19 bacterial agents [1]. In the United States, 2.1 to 2.4 million foodborne disease cases were attributed to human Campylobacteriosis, with 99 fatalities [2]. Mead et al. [1] reported that Campylobacter cost $1.2 billion of the $6.9 billion allotted annually for foodborne illnesses. Campylobacter is common in poultry, meat, unpasteurized milk, untreated water, and fish (Center for Disease Control, 1997) [3]. Poultry is the primary vehicle transmitting Campylobacter to humans (Badger et at., 2004). In the United States, 69% of the chickens bought from a local supermarket were contaminated with Campylobacter jejuni [4].
Campylobacter is a gram-negative, microaerophilic, thermophilic spiral bacteria and grows best at 42 °C. Campylobacter is sensitive to many environmental factors, such as high temperatures, high oxygen, freezing, drying, disinfectant, acid conditions (pH<5.0), and salinity [5]. Campylobacter jejuni (C. jejuni), and to a lesser extent, Campylobacter coli (C. coli) are the species most commonly associated with disease in humans [6]. In a preliminary study, Hume et al. [7] stated that there appeared to be a change in the growth profile of the two strains, C. jejuni, and C. coli, from the pre-harvest to the post-harvest stage. They reported that C. jejuni appeared in this study to be the dominant strain in the pre-harvest stage, whereas the C. coli strain is more prevalent in the post-harvest stage. The change in the growth profile may create significant problems in the control of this foodborne pathogen.
Many factors influence the transmission of Campylobacter in poultry. Among them are horizontal Jacobs R [8] or water systems [9], and to a lesser extent, vertical transmission [10]. Campylobacter Colony-Forming Unit (CFU) was highest in ceca and colon of birds at slaughter [11]. Campylobacter was observed in broiler carcass’s respiratory tract before and after commercial scalding [11]. These observations emphasize the importance of pre-harvest treatment in the control of Campylobacter.
Attempts have been made to reduce Campylobacter from the farm gate to the consumer table. Line [12] incorporates aluminum sulfate and sodium in the contaminated litter to control the pathogen at the pre-harvest stage Byrd et al. [13] fed lactic acid to broilers in the drinking water before transportation. Washing the carcasses with Trisodium Phosphate (TSP) and sodium chlorite have also been tried [14]. All the above methods have somehow reduced the Campylobacter levels. To effectively reduce the contamination level on processed broilers, pathogen-free or nearly pathogen-free birds must be delivered to the processing plant [15].
Commonly human infections due to Campylobacteriosis is treated with antibiotics. Antibiotics have come under increasing scrutiny by some scientists, consumers, and government regulations, because of the potential development of antibiotic resistance bacteria, including pathogenic strains, after prolonged use of antibiotics [16]. The ban of some antibiotics used in the poultry industry stimulated the search for natural and viable alternatives to fight diseases [17]. Due to consumer concern, the poultry industry is looking for other options to antibiotic-based drugs and also antibiotic-free growth enhancement feed additives [18]. The use of Yeast Culture Residue (YCR, Bio-Mos, Alltech INC) as an alternative to improve performance and residue diseases in livestock is well documented [19-21]. Bio-Mos, an oligosaccharide, contains mannan, a complex carbohydrate found in the yeast sac’s outer cell wall (Saccharomyces cerevisiae) [21]. Yeast culture residue has been used as an alternative to antibiotic-based drugs in feed for enhancing the performance of broilers infected by a coccidia diet [19] and by controlling aflatoxicosis [18]. A Pathogen binds to the mannan instead of the intestinal wall and is flushed out of the gastrointestinal tract without causing any severe damage to the host [22]. The yeast acts to stabilize and protect the intestinal tract from colonization by opportunistic pathogens until the normal protective microflora can be developed or restored [20].
With the emphasis on finding alternatives to antibiotic -based drugs in treating foodborne pathogens, it was hypothesized that YCR could reduce the cecal population of Campylobacter by blocking its attachment of the epithelial cells.
Objectives
To examine the sensitivity of selected strains of Campylobacter (C. jejuni and C. coli) to the dietary inclusion of YCR during the preharvest stage.
Materials and Methods
Birds and Feeding Treatments
Four hundred Cornish Rock unvaccinated and unsexed commercial day-old broiler chickens were purchased from Tyson hatchery in Gonzales, Texas. They were assigned one hundred per pen to each of the four treatment groups. Fecal materials from the shipment boxes were collected and tested for Campylobacter. All litter samples were negative for the pathogen.
The chicks were brooded at 32.2 °C, which was decreased by -15 °C weekly, to a low of 23.8 °C at week 6. The chicks were housed on concrete floors, covered with pine sawdust litter, to a depth of 4 inches. To minimize cross-contamination, a footbath was placed at the door of each pen. For additional security, the negative control birds in the pens with Campylobacter were always attended to first.
The experiment was designed as a completely randomized factorial consisting of four treatment groups. Each treatment group was assigned one hundred birds per pen. The treatment was Treatment 1: control- uninfected and untreated; Treatment 2: Campylobacter infected and untreated; Treatment 3: YCR treated and uninfected; and Treatment 4: Campylobacter infected and treated with YCR. At 2kg/ton, YCR was added to the starter and finisher feed and mixed, using a commercial mixer, for fifteen minutes for even distribution. The feed, provided ad libitum in a mash form, was labeled for each treatment and stored daily in plastic barrels. The feed was formulated from a standard cornsoybean based commercially formulated ration, recommended by the National Research Council (NRC, 1994) [23]. The starter ration consisted of 21% crude protein and 3300kcal/kg of M.E. energy and was fed for the first two weeks. The finisher ration consisted of 19% Crude Protein (CP) and 3300kcal/kg of M.E. energy and fed from weeks three to six weeks. Water was available for ad libitum consumption.
Campylobacter
Ten strains of Campylobacter spp. ( C. jejuni: Pr 1-1, Pr 1-3, Pr 1-7, Pr 1-24, Pr 5-1; C.coli : Pr 5-3, Po 1-1, Po1-5, Po5-5, Cr 1-4) isolated from chickens were used for the cocktail preparation. The strains were stored in 20% glycerol broth at -70° C. After thawing the samples, a sterile plastic loop was used to inoculate each species to the Campy-Cefex agar plates [24]. The plates were later incubated at 42° C for 48 hours micro-aerobically (85% nitrogen, 10% carbon dioxide, and 5% oxygen). A sterile plastic loop (10L) was used to transfer a single Campylobacter colony from each plate into Campylobacter enrichment broth and incubated micro aerobically at 42°C for 48 hours. From the 10ml in each test tube, 0.5ml of culture was placed in 30ml of Bolton broth for each species and then combined, resulting in a 300ml cocktail stock. The infected chicks were challenged with a cocktail mixture (108 CFU) of ten strains of Campylobacter spp. (5 C. jejuni and 5 C. coli strains). Infected chicks were challenged by crop gavage at 1-day-old.
Ten birds were sacrificed by cervical dislocation and submerged in P -128 disinfectant. The birds were dissected, and the ceca were aseptically removed. A total of 0.4 grams of cecal content was collected into labeled sterile test tubes containing 3.6 ml of sterile Butterfields buffer to make a 4.0ml volume of a 1:10 dilution. Before volatile fatty acids (acetic, propionic, and butyric) and lactic acid analyses, the samples were centrifuged at 8000 x rpm for 10 minutes in an R.T. (Beckman GS-6R) followed by measuring of pH (Fisher Scientific AR 15) and then stored at 4 °C until they were analyzed.
A sterile pipette was used to place 0.1ml of serially diluted sample from the 10-3 to 10-5 dilution (Campylobacter group) on to Campy-Cefex agar plates that were placed in plastic bags and incubated micro aerobically for 48 hours at 42° C. Campylobacter CFU were counted and transformed to log10 CFU per gram of cecal content.
Data Collection and Statistical Analysis
Analyses for acetic, propionic, and butyric acids were expressed in mole/g of cecal content. The samples for acetic, propionic, and butyric acids were analyzed on Shimadzu G.C. 14A gas chromatography using an AOI-20 injector fitted with Carbopack B-DAY (Supelco, Inc, Bellefonte, PA) glass column. The lactic acid concentration was determined according to the method of Hohorst [25], with Ciba Corning (550 Express Plus) and expressed in mole/g of cecal content.
Data for pH, microbial population (CFU), lactic acid, and volatile fatty acids were subject to analysis of variances (ANOVA) as a 2x2 factorial using the General Linear Models procedures of SAS (1998) [26]. Duncan New Multiple Range Test was used to compare mean differences at the 5% probability level (P<0.05). GLM procedure was applied to analyzed microbial data for the colony-forming unit of Campylobacter.
Results and Discussion
Yeast culture residue significantly (P<0.05) reduced the Campylobacter population in the cecal content of broilers at 2,4 and 6 weeks of age (Figure 1-3). Compared to the untreated and infected birds, the concentrations of Campylobacter isolated from the ceca were significantly (P<0.05) reduced weekly by YCR. Yeast culture residue reduced cecal Campylobacter colonization by 6.89% at week 2, by 2.04% at week 4, and 45.49% at week 6. The treatment with YCR significantly (P<0.05) decreased the cecal Campylobacter population at week 6 by a 1.92 log. The effect of YCR on the reduction of Campylobacter colonization increased over time. The data suggested that the concentration of Campylobacter per gram of cecal content remained relatively the same over time (Figure 1-3). Campylobacter population present at Week 6 probably represents the most critical observation as those levels reflect the pathogen loads that the birds would likely carry to the processing plant [12]. The higher the incidence of the pathogen in the ceca, the more significant is the possibility of survival on the carcass exterior [27].
Figure 1:Effects of yeast culture residue and Campylobacter on ceca; CFUa in Broilers ad 2 weeks of age.
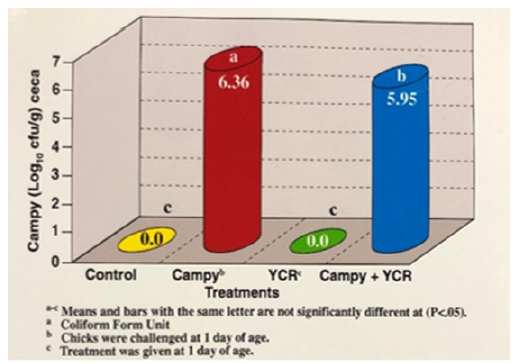
Figure 2:Effects of yeast culture residue and Campylobacter on ceca; CFUa in Broilers ad 4 weeks of age.

Figure 3:Effects of yeast culture residue and Campylobacter on ceca; CFUa in Broilers ad 6 weeks of age.

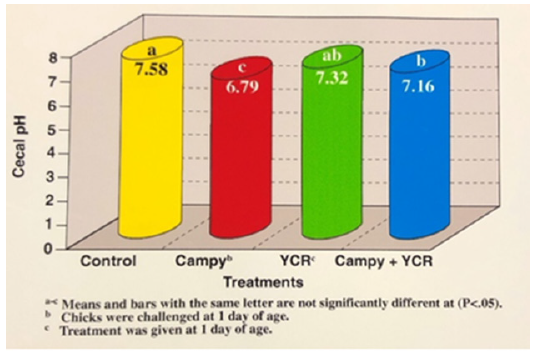
The significant decrease in Campylobacter’s cecal population could be due to the substantial increase in pH level and the reduction of lactic acid in the birds treated with YCR (Figures 4 and 5). Mean lactic acid concentrations increased from 0.62 to 0.85umol/g of cecal content in the control and Campylobacter treatment, respectively. Birds fed YCR that was not infected had the lowest lactic acid concentration within the ceca. The YCR appears to be improving the establishment of natural microflora within the gastrointestinal tract.
Figure 4:Effects of yeast culture residue and Campylobacter on cecal lactic acid in broilers at 6 weeks of age.
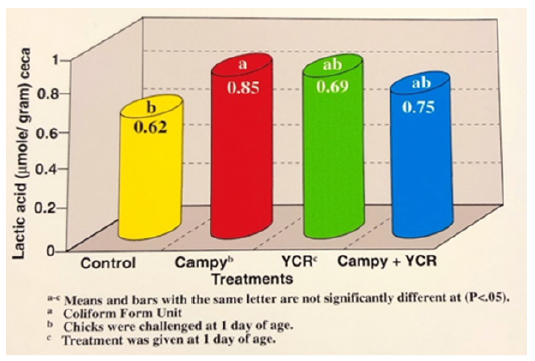
Figure 5:Effects of yeast culture residue and Campylobacter on cecal pH in broilers at 6 weeks of age.
The volatile fatty acids (acetic, propionic, and butyric) (Table 1) were lowered by YCR, whereas the Campylobacter infected and untreated birds had the highest cecal volatile fatty acids concentration.
Table 1:Effects of Yeast Culture Residue (YCR) on cecal Campylobacter volatile fatty acids in broilers at 6 weeks of age.
a-cmeans ±SD with the same letters within columns are not
significantly (P<05).
dchicks were challenged at 1 day of age.
etreatment began when chicks were 1 day of age.
Conclusion
Yeast culture residue (Bio-Mos) has been shown to significantly reduce CFU colonization, decrease lactic levels, and increase pH within the ceca at the pre-harvest stage. Bio-Mos is a natural product, environmentally friendly, and does not require a withdrawal time when used as a feed supplement to boilers. Bio-Mos is cost-effective, and it can be easily added to the ration. Compared to antibiotics, there is no available data indicating that Bio-Mos can lead to illness in a human. Therefore, Bio-Mos incorporated in broiler birds’ ration could stabilize and protect the intestinal tract from colonization of Campylobacter, thus serving as a viable alternative to in-feed antibiotic-based drugs.
Acknowledgment
The authors wish to acknowledge the contributions of Alltech Biotechnology Corp for supplying the yeast culture residue and USDA-ARS, College Station, Texas, for providing the Campylobacter.
References
- Mead PS, Slutsker VD, McCaig LF, Bresee JS, Shapiro C, et al. (1999) Food-related illness and death in the United States. Emerging Infectious Disease 5(5): 607-625.
- Tauxe RV (1992) Epidemiology of Campylobacter jejuni infected in the United States and other Industrialized Nation. In: Nachamkin I, Blaster MJ, Tompkins S, (Eds.), Campylobacter jejuni current and future trends. American Society for Microbiology, Washington, USA, pp. 9-19.
- Center for Disease Control (1997) Emerging Foodborne Disease. Emerging infectious Disease No 3. Center for Disease Control and Prevention, Atlanta, GA, USA.
- Willis WL, Murray C (1997) Campylobacter jejuni seasonal recovery observations of retail market broilers. Poultry Science 76(2): 314-317.
- Altekruse SF, Stern NJ, Fields PI, Swerdlow DL (2000) Campylobacter jejuni S. Food and Drug Administration, Blacksburg, Virginia, USA.
- Wilm KH (2003) Our Food. Database of food and related sciences.
- Hume ME, Byrd AJ (2000) The genetic pattern of Campylobacter isolation from market-age broiler crops at pre- and post-feed withdrawal and carcass rinses. Poultry Science Association. Postal 79 (Supplement 1) 5.
- Jacobs-Reitsma WF (1997) Aspects of epidemiology if Campylobacter in poultry. Vet Q 19(3): 113-117.
- Pearson AD, Greenwood MH, Healing TD, Rollins D, Shahamat M, et al. (1993) Colonization of broiler chickens by waterborne Campylobacter jejuni. Appl Environ Microbiol 59(4): 987-996.
- Pearson AD, Greenwood MH, Feltham RK, Healing TD, Donaldson J, et al. (1996) Microbial ecology of Campylobacter jejuni in a United Kingdom chicken supply chain: Intermittent familiar sources, Vertical transmission, and Amplification by flock propagation. Appl Environ Microbiol 62(12): 4614-4620.
- Berrang ME, Meinersmann RJ, Buhr RJ, Reimer NA, Phillips RW, et al. (2003) Presence of Campylobacter in the respiratory tract of broiler carcasses before and after commercial scalding. Poultry Science 82(12): 1995-1999.
- Line JE (2002) Campylobacter and Salmonella Populations are associated with chickens raised on acidified litter. Poultry Science 81(10): 1473-1477.
- Byrd JA, Hargis BM, Caldwell DJ, Bailey RH, Herron KL, et al. (2001) Effects of lactic acid administration in the drinking water during preslaughter feed withdrawal on Salmonella and Campylobacter contamination of broilers. Poultry Science 80(3): 278-283.
- Bashor MP, Curtis PA, Keener KM, Sheldon BW, Kathariou S, et al. (2004) Effects of carcass washers on Campylobacter contamination in large broiler processing plants 83(7): 1232-1239.
- Bailey JS (1993) Control of Salmonella and Campylobacter in poultry production. A summary of work at Russell Research Center. Poultry Science 72(6): 1169-1173.
- Ratcliff J (2000) Antibiotic bans- a European perspective. Pages 135-152 in Proceedings of the 47th Maryland Nutrition Conference for Feed Manufacturers.
- Revington B (2002) Feeding poultry in the post-antibiotic era. Multi-State Poultry Meeting. Indianapolis, Indiana, USA.
- Stanley VG, Ojo R, Woldesenbet S, Hutchinson HD, Kubena LF (1993) The use of Saccharomyces cerevisiae to suppress the effect of aflatoxicosis in broiler chicks. Poultry Science 72(10): 1867-1872.
- Stanley VG, Gary C, Daley M, Krueger WF, Sefton AE (2004) An alternative to antibiotic-based drugs in feed for enhancing the performance of broilers grown on Eimeria spp. -infected litter. Poultry Science 83: 39-44.
- Line EJ, Bailey JS, Stern NJ (1997) Yeast treatment to reduce Salmonella and Campylobacter population associated with broiler chickens subjected to transport stress. Poultry Science 76(9): 1227-1231.
- Oyofo BA, Droleskey RE, Norman JO, Mollenhauer HH, Ziprin RL, et al. (1989b) Inhibition by mannose of in vitro colonization of the small chicken intestine by Salmonella typhimurium. Poultry Sci 68(10): 1351-1356.
- Newman K (1994) Mannan-oligosaccharides: Natural Polymers with a significant impact on the gastrointestinal microflora and the immune system. In: Lyons TP, Jacques KA (Eds.), Biotechnology in the Feed Industry: Proceedings of Alltech Tenth Annual Symposium, Nottingham University Press, Nottingham, UK, pp. 167-174.
- National Research Council (NRC) (1994) Nutrients requirement for poultry. (8th revised edn), National Academy Press, Washington, DC, USA.
- Stern NJ, Wojton B, Kwiatek K (1992) A differential- selective medium and dry ice-generated atmosphere for the recovery of Campylobacter jejuni. J Food Prot 55(7): 514-517.
- Hohorst HJ (1965) Lactate. In: Bergmeyer HV (Ed.), Methods of enzymatic analysis, Academic Press, New York, USA, pp. 266-270.
- SAS Institute (1998) SAS/STAT Guides for Personal Computers. (8th edn), SAS Institute Inc., Cary, NC, USA.
- Stanley VG, Ojo R, Woldesenbet S, Hutchinson HD, Kubena LF (1993) The Use of Saccharomyces cerevisiae to suppress the effect of aflatoxicosis in broiler chickens. Poultry Science 72(10): 1867-1872.
© 2022 Milton Daley. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






