- Submissions

Full Text
Clinical Research in Animal Science
Extract of Sweet cherries from Campania Region Possesses Antidiabetic Properties Modelling the Serum Insulin, Adiponectin, Leptin, and Resistin in Diabetic Rats
Rosaria Sciarrillo1*, Vito Gallicchio2, Mariana Di Lorenzo3, Maria De Falco3,4,5 and Carmine Guarino1
1Department of Science and Technologies, University of Sannio, Via De Sanctis sncI-82100 Benevento, Italy
2Vascular Surgery, Hospital of National Importance San Giuseppe Moscati, Via Contrada Amoretta, 83100 Avellino, Italy
2Department of Biology, University of Naples “Federico II”, Naples, Italy
2National Institute of Biostructures and Biosystems (INBB), Rome, Italy
2Center for Studies on Bioinspired Agro-Environmental Technology (BAT Center), Portici, Italy
*Corresponding author:Rosaria Sciarrillo, Department of Science and Technologies, University of Sannio, Via De Sanctis sncI-82100 Benevento, Italy
Submission: November 12, 2021;Published: November 23, 2021

ISSN: 2770-6729Volume 2 - Issue 1
Abstract
The obesity-associated diseases, e.g. diabetes, are spread worldwide. The polyphenols have Interesting biological activities such as anti-inflammatory, antioxidant, anti-allergic, hepatoprotective, anti-thrombotic, antiviral and anti-carcinogenic activities. These constituent nutrients and bioactive food components are present in sweet cherry. Cherries are thought to have beneficial health properties, moreover, a diet with antioxidant-rich cherries significantly lowers the insulin, fasting glucose levels, key markers for the development of diabetes. In particular, within the Campania region (Italy), several sweet cherry local cultivars have been studied. Two accessions (Patanara and Mulegnana Nera) of autochthonous germplasm of Campania, which have significant differences in phenols, flavonoids and anthocyanins levels, are used to evaluate their extracts antioxidant effect in alloxan-induced diabetic rats. This study was designed to evaluate the consequence of antioxidant extracts from cherries on the blood glucose, cholesterol and triglycerides and serum insulin, leptin, adiponectin and resistin concentrations after 30 days of intragastrical administration of extracts of “Patanara” and “Mulegnana Nera” in alloxan-induced diabetic rats. Administration of “Patanara” and “Mulegnana Nera at a concentration of 200mg/kg body weight for 30 days, significantly reduced the levels of blood glucose and serum insulin, leptin and adiponectin levels. In conclusion, in this study, cherry antioxidant extract showed a beneficial effect on the diabetic rats.
Keywords:Sweet cherry; Antioxidant; Blood glucose; Diabetes; Leptin
Introduction
Diabetes is an endocrine metabolic syndrome featured by higher levels of blood glucose [1,2]. Therefore, it is necessary to maintain blood glucose levels lower to reduce the serious complications in various organs [2]. Even if several tissues as pancreas, liver, brain, intestine, adipose and muscle tissue control the normal blood glucose levels, pancreas has an important role in glucose homeostasis [3].
In recent times, many studies have been concentrated on some food factors that may be beneficial for reducing the risk of metabolic syndrome. There has been evidence that food itself is directly beneficial for the improvement of the metabolic dysfunction [2]. In fact, fruits and vegetables have a protective role against the development of human diseases such as diabetes because they are rich in simple sugar (glucose, fructose, sucrose and sorbitol), hydro soluble (C,B) and lipo-soluble (A, E and K) vitamins, minerals, mainly potassium, phenolic compounds (flavonoids and anthocyanins) [4]. Sweet cherry is one of the greatest popular fleshy non-climacteric spring-summer seed fruit species belonging to the genus Prunus, within the Rosaceae family [5].
Italy is the fourth producer in the world and the first in Europe and Campania region is primarily producer [6]. Several studies showed that daily intake of sweet cherries reduce gout and arthritis pain, neurological, gastrointestinal, tumoral and cardiovascular diseases [7,8] and prevents diabetes [9,10]. In fact, the anthocyanins provoke beneficial effects in diabetes by acting on various molecular targets, regulate different signaling pathways in multiple organs and tissues, such as liver, pancreas, kidney, adipose, skeletal muscle system, and brain [11]. Several works highlight the importance of the endogenous antioxidant defense system in relation to the fruit quality and to the shelflife [12-14].
Recently, the autochthonous sweet cherry cultivars of southern Italy (Campania Region) have been characterized by the presence of bioactive molecules exhibiting antioxidant properties such as total ascorbate, carotenoids and xanthophyll, phenolic and anthocyanins [15]. In the light of the foregoing, among the autochthonous germplasm of Campania studied [15], we chose two accessions (Patanara and Mulegnana Nera) which have significant differences in the levels of phenolic, flavonoids and anthocyanins to evaluate the antioxidant effect of their extracts in alloxan-induced diabetic rats. In fact, it was observed that the concentration of total level of ascorbate (AsA) in “Patanara” was 126.34μg g−1 fresh weight [FW] while in “Mulegnana Nera” was 158.99μg g−1 FW, showing that these accessions of Campania cherries are a good source of ascorbate for human nutrition, in relation with mean levels of 60-100μg g−1 FW previously reported [15]. As for the level of total phenolics, these cultivars exhibited significant variations, with average fruit content ranging from 8.46mg micrograms of gallic acid equivalents (GAE) g−1 FW in Patanara to 16.25mg GAE g−1 FW in Mulegnana Nera [15]. The most abundant phenolic acids in sweet cherries are hydroxycinnamic acids, whereas the major flavonoids subclasses are anthocyanins and flavanols.
As for anthocyanins, significant differences were found among cultivar Patanara (4.80μg g−1 FW) showing the lowest fruit levels and Mulegnana Nera (360.90μg g−1 FW) expressing the highest accumulation in the fruit flesh and skin [15]. In sweet cherry the anthocyanins concentration is the most important indicator of fruit quality driving consumer appeal, because of both their antioxidant activity and their role in determining skin and flesh colour. Major anthocyanins were cyanidin 3-O-sophoroside and pelargonidin 3, 5-O-diglucoside, whereas the major non-anthocyanins phenolic compound in Mulegnana Nera were coumaroylquinic acid and neochlorogenic acid [15]. The Trolox equivalent antioxidant capacity (TEAC) assay showed that Patanara (1.01 nmol Trolox equiv.mg−1 FW) expresses the lowest activity, and Mulegnana Nera (1.69 nmol Trolox equiv. mg−1 FW) has the highest activity [15].
This is the reason why we evaluated the serum concentrations of glucose, cholesterol and triglycerides and insulin, leptin, adiponectin and resistin after 30 days of intragastrical administration of extracts of Prunus avium in alloxan-induced diabetic rats. The object of the present study was to search in vivo the consequence of a 30-days administration with extracts of two accessions (Patanara and Mulegnana Nera) of the autochthonous germplasm of Campania on several risk factors for diabetes.
Material and Methods
Sample collection and Preparation of fruits extract From the regional experimental farm “Improsta”, Eboli Campania (Italy), sweet cherry fruits of two varieties (“Mulegnana Nera” and “Patanara) were gathered at commercial maturity. The tested accessions were “Mulegnana Nera” and “Patanara” because they showed different bioactive compounds [5]. Samples of sweet cherry fruits were cleaned with distilled water and frozen at -18 °C until use. All solvents and reagents were purchased from commercial sources at a high grade of purity and used as received unless stated otherwise. After the thawing, 20mL ethanol-acid solvent (70%) was added to each sample (1000g) and the mixture was blended for overnight. The supernatant were filtered through a Buchner funnel vacuum and Whatman No.1. The filtered product was then placed in a balloon container to separate the ethanol-acid solvent via a vacuum evaporator at 35 °C to have a concentrated extract with total soluble solid content equal to 60 °Brix. After solvent evaporation, the resulted pure cherry extract was collected as the concentrated substance in the bottom of the container and used directly in the process of treatment [16].
Treatment of animals in vivo
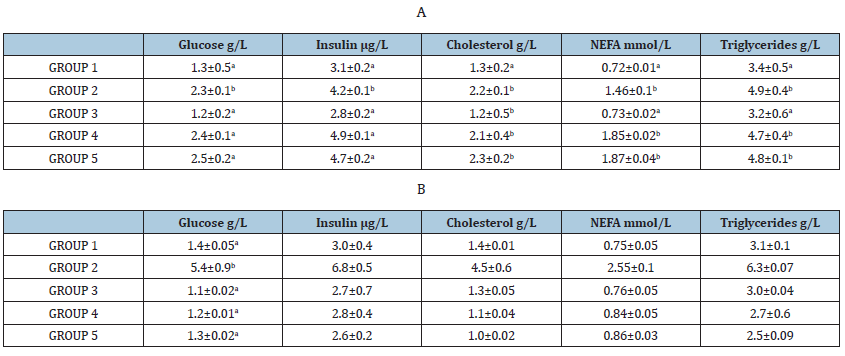
This study was carried out in accordance with the principles of the National Guide for the Care and Use of Laboratory Animals. The Italian Ministry of Health approved the protocol (Table 1A). Twentyfive Wistar rats with an average weight of 200±20g were naturalize to laboratory conditions (temperature: 25±2 °C) and maintained on 12-hour light:12-hour dark cycle and they were given standard diet and had free accesses to food and water ad libitum throughout the study (Table 1B).
Table 1:Effect of ethanolic extracts of accessions of sweet cherry on blood glucose, cholesterol and triglycerides, insulin and NEFA level in induced diabetes in rats before (A) and after 30 days (B). Values are mean ± SEM in g/L. *p < 0.05; **p < 0.001.
After one week of adaption, rats were casual separated
into five groups (n=5). Four groups (n=5) of rats were injected
intragastrically with a single dose of 120mg/kg i.p. of alloxan
monohydrate (Sigma Chemical Company Inc.) dissolved in sterile
normal saline solution. The animals that had blood glucose levels
of above 200mg/dl after 72 hours of alloxan injection were used
for the study; whereas the control animals received physiological
saline (200mg/kg). In particular:
A. Group 1 Normal Rats treated intragastrically with physiological
saline solution (200mg/kg) for 30 days.
B. Group 2 Diabetic Rats treated intragastrically with
physiological saline solution (200mg/kg) for 30 days.
C. Group 3 Diabetic rats treated with a single subcutaneous
injection of 1 unit of NPH-insulin for 30 days.
D. Group 4 Diabetic rats treated intragastrically with ethanolic
extracts (200mg/kg) of Prunus avium accession “Mulegnana
Nera” for 30 days.
E. Group 5 Diabetic rats treated with ethanolic extracts (200mg/
kg) intragastrically of Prunus avium accession “Patanara” for
30 days.
The rats were weighed every week. The fasting blood samples were collected on days 1, 7, 14, 21 and 28 to determine the glucose level. At the end of the treatments, after 4 weeks, rats were feeddeprived overnight, anesthetized by exposure to CO2, and killed by decapitation. Blood samples were centrifuged immediately, and the serum samples were stored at -20 °C until analysis of specific hormonal data. The analysis was carried out before the treatments (T0) and after four weeks from administration of extracts (TF).
Hormone assays
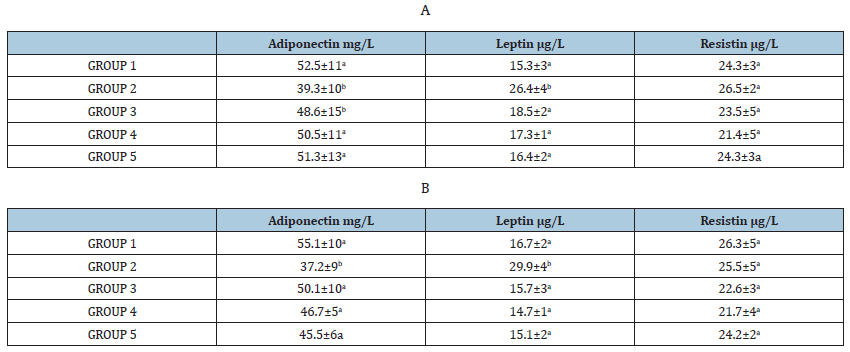
Glucose (Gluco-Quant, Roche, Mannheim, Germany), insulin (Rat insulin ELISA, Millipore, Schwalbach, Germany), cholesterol (CHOD-PAP, Roche) and triglycerides (GPO-PAP, Roche) were determined using commercial kits according to the manufacturers’ instructions. The adiponectin, insulin, leptin, and resistin were quantified in serum. The level of adiponectin was measured by a commercially kit RIA (Linco Research; intra- and interassay CVs, 4.8 and 7.3%, respectively). Insulin concentrations were measured by a commercial radioimmunoassay [RIA; Sorin, Biomedica, Saluggia, Italy; intra- and interassay CVs, 6.6 and 6.2%, respectively]. Leptin levels were measured by using a polyclonal antibody RIA raised in rabbits against highly purified recombinant human leptin (Linco Research, St Louis, MO, USA; intra and interassay CVs, 4.8 and 3.5%, respectively). Resistin level was measured by using a sandwich enzyme-linked immunosorbent assay (ELISA; Phoenix Pharmaceutical, Inc, Belmont, CA, USA; intra- and interassay CVs, 3.4 and 6.3%, respectively) (Table 2A).
Statistical Analysis
Obtained data are showed as mean ± SEM and were analyzed using Analysis of Variance (ANOVA). Means were separated by using the Tukey Multiple Range Test. Differences were considered significant when p<0.05 and p<0.001 (Table 2B).
Table 2:Effect of ethanolic extracts of accessions of sweet cherry on blood Adiponectin, Leptin and Resistin level in induced diabetes in rats before (A) and after 30 days (B). Values are mean ± SEM in g/L. *p < 0.05; **p<0.001.
Result and Discussion
The incidence of sweet cherry extracts on the blood glycaemia of experimental animals was determined after 30 days of administration (Figure 1). There was a significant increase in the blood glucose level in diabetic rat (p<0.05) compared to the control group. Extracts significantly lowered the blood glucose concentration after 30 days but the sweet cherry extracts did not show completely anti hyperglycemic effect as the insulin administration with which after 30 days of treatment; the blood glucose level tends to be normal. Therefore, administration of sweet cherry extracts and insulin tended to bring this parameter within normal ranges. The observed effect of accession “Mulegnana Nera” was significantly better than the effect of accession “Patanara”. Our study indicates that both accessions of sweet cherry extracts have anti-diabetic activity. Ethanol extracts of sweet cherries exhibited significant antihyperglycemic activities in alloxan-induced hyperglycemic rats. Besides, this difference between “Mulegnana Nera” and “Patanara” could be attributed to the different content of anthocyanins. Anti-diabetic properties are assumed for anthocyanins and human studies as well as animal ones, reported increased insulin sensitivity or lower blood glucose concentrations after an anthocyanin administration [16,17]. Therefore, this effect of anthocyanin of sweet cherries extracts could act in two ways, on one hand, lower the glucose level in diabetic rats and prevent the destruction of beta cell in the pancreas, on the other hand, increasing insulin secretion recovering the function of damaged beta cell [16]. In fact, increased insulin levels was observed in diabetic rats (Figure 2) that were treated with extract of Prunus avium. Though the process is unclear, it is possible to conjecture that the increased concentrations of insulin to stimulation of cell and/or an increased release of insulin from the remnant beta cells. The increased levels of blood glucose were seen to be substantially lowered in the treated group of extracts of sweet cherry, compared to diabetic rats. Therefore, these levels were not lowered to the same magnitude compared to control group. The most abundant anthocyanins pigments present in Mulegnana Nera was cyanidin 3-O-sophoroside that is the best known and most investigated anthocyanin. Cholesterol concentrations were reduced after 30 days of sweet cherries extracts administration (Figure 3). Sweet cherries extracts also tended to decrease triglyceride concentrations (p <0.001) (Figure 2) [18]. Our results indicate that administration of extracts of Mulegnana Nera can decrease serum concentrations of cholesterol and triglycerides. One possible mechanism for cholesterol lowering effects of anthocyanins could be the inhibition of cholesterol synthesis [17].
Figure 1:Effect of ethanolic extracts of accessions of sweet cherry on blood glucose level in induced diabetes in rats after 30 days. Values are mean ± SEM in g/L. *p<0.05; **p<0.001.
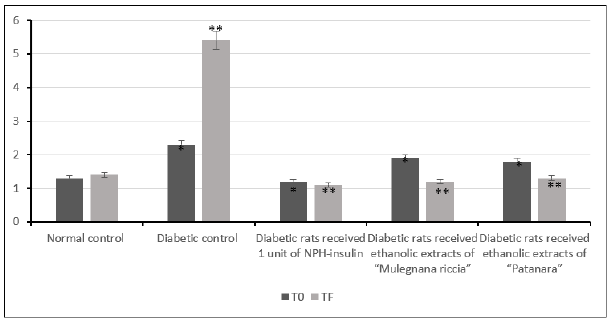
Figure 2:Effect of ethanolic extracts of accessions of sweet cherry on serum Insulin, Leptin and Resistin level in induced diabetes in rats after 30 days. Values are mean ± SEM in μg/L; *p<0.05; **p<0.001.
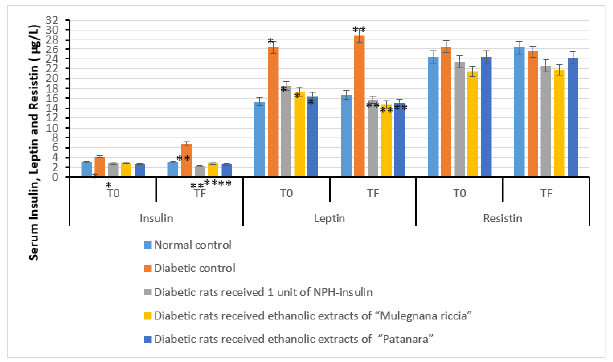
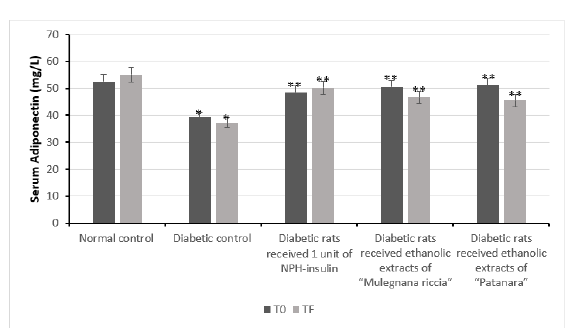
The adipose tissue secretes adipokines (e.g. adiponectin, leptin, and resistin), which governing the metabolism. The adiponectin, leptin, and resistin were quantified in serum. High levels of leptin and resistin, occurring in obese individuals, promote the development of insulin resistance, whereas adiponectin seems to prevent insulin resistance. Diabetic rats treated with extracts of sweet cherry had lower serum leptin (Figure 4) and adiponectin levels (Figure 3) compared to the control group. Resistin concentrations did not vary between diabetic rats treated with extracts of sweet cherry and control group (Figure 4). The administration of extracts of Prunus avium decreased levels of leptin and adiponectin, whereas no result was observed for resistin. Data on the influence of anthocyanins on adipokines are rather controversial. Further studies are needed to determine which adipose depots are responsible for the observed changes in serum and under which conditions anthocyanins exert this effect.
Figure 3:Effect of ethanolic extracts of accessions of sweet cherry on serum cholesterol and triglycerides level in induced diabetes in rats after 30 days. Values are mean ± SEM in g/L; *p<0.05; **p<0.001.
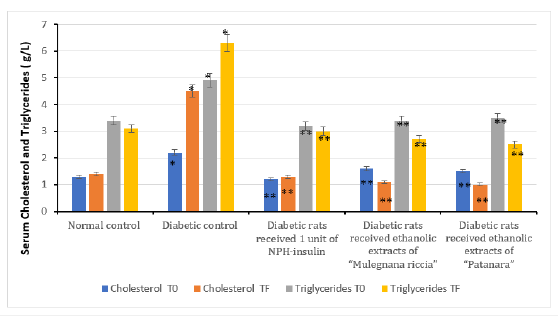
Figure 4:Effect of ethanolic extracts of accessions of sweet cherry on serum Adiponectin level in induced diabetes in rats after 30 days. Values are mean ± SEM in mg/L. *p<0.05; **p<0.001.
Conclusion
In the present study, it was investigated the influence of an extract of sweet cherry on lipid metabolism, serum concentrations of adipokines. In conclusion, a 30 days administration of extracts of sweet cherry decreases in serum cholesterol, leptin, and adiponectin levels. These data demonstrated that intake of extracts, might have a preventive potential for obesity-associated diseases such as diabetes or cardiovascular diseases.
References
- Gowd V, Nandini CD (2015) Erythrocytes in the combined milieu of high glucose and high cholesterol shows glycosaminoglycan-dependent cytoadherence to extracellular matrix components. International Journal of Biological macromolecules 73: 182-188.
- Gowd V, Jia Z, Chen W (2017) Anthocyanins as promising molecules and dietary bioactive components against diabetes - A review of recent advances. Trends in Food Science & Technology 68: 1-13.
- Röder PV, Wu B, Liu Y, Han W (2016) Pancreatic regulation of glucose homeostasis. Experimental and Molecular Medicine 48(3): e219.
- Pacifico S, Di Maro A, Petriccione M, Galasso S, Piccolella S, et al. (2014) Chemical composition, nutritional value and antioxidant properties of autochthonous Prunus avium cultivars from Campania Region. Food Research International 64: 188-199.
- Mirto A, Iannuzzi F, Carillo P, Ciarmiello LF, Woodrow P, et al. (2018) Metabolic characterization and antioxidant activity in sweet cherry (Prunus avium) Campania accessions Metabolic characterization of sweet cherry accessions. Food Chemistry 240: 559-566.
- FAO (2013) FAO statistical yearbook 2013: World food and agriculture.
- Ferretti G, Bacchetti T, Belleggia A, Neri D (2010) Cherry antioxidants: From farm to table. Molecules 15(10): 6993-7005.
- Duarte AP, Silva B M (2014) Nutritional and phytochemical potential of “Prunus avium ”. In: Gupta VK (Ed.), Natural products: Research reviews, M/S Daya Publishing House, Nova Deli, India, 4: 85-202.
- Teixeira R, Silva LR (2013) Bioactive compounds and in vitro biological activity of Euphrasia rostkoviana Hayne extracts. Industrial Crops and Products 50: 680-689.
- Silva LR, Teixeira R (2015) Phenolic profile and biological potential of Endopleura uchi Asian Pacific Journal of Tropical Medicine 8(11): 889-897.
- Lee JS, Kim YR, Song IG, Ha SJ, Kim YE, et al. (2015) Cyanidin-3-glucoside isolated from mulberry fruit protects pancreatic beta-cells against oxidative stress-induced apoptosis. International Journal of Molecular Medicine 35: 405-412.
- Dyduch Siemińska M, Najda A, Dyduch J, Gantner M, Klimek K (2015) The content of secondary metabolites and antioxidant activity of wild strawberry fruit (Fragaria vesca). Journal of Analytical Methods in Chemistry 2015: 831238.
- Matthes A, Schmitz Eiberger, M (2012) Polyphenol content and antioxidant capacity of apple fruit: Effect of cultivar and storage conditions. Journal of Applied Botany and Food Quality 82(2):152-157.
- Zhang Y, de Stefano R, Robine M, Butelli E, Bulling K, et al. (2015) Different ROS-scavenging properties of flavonoids determine their abilities to extend shelf life of tomato. Plant Physiology 169(3): 1568-1583.
- Di Matteo A, Russo R, Graziani G, Ritieni A, Di Vaio C (2017) Characterization of autochthonous sweet cherry cultivars (Prunus avium L.) of southern Italy for fruit quality, bioactive compounds and antioxidant activity. J Sci Food Agri 97(9): 2782-2794.
- Lachin T (2014) Effect of antioxidant extract from cherries on diabetes. Recent Patents on Endocrine, Metabolic & Immune Drug Discovery 8(1): 67-74.
- Graf D, Seifert S, Jaudszus A, Bub A, Watzl B (2013) Anthocyanin-rich juice lowers serum cholesterol, leptin, and resistin and improves plasma fatty acid composition in fischer rats. Plos One 8(6): e66690.
- Liu Y, Li D, Zhang Y, Sun R, Xia M (2014) Anthocyanin increases adiponectin secretion and protects against diabetes-related endothelial dysfunction. Am J Physiol Endocrinol Metab 306(8): E975-E988.
© 2021 Rosaria Sciarrillo. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






