- Submissions

Full Text
Clinical Research in Animal Science
Determination of Tannin levels and Trypsin Inhibition in Selected Legume Grains and their Effects on Rabbit Pathophysiological Condition
Chisowa DM*
Southern University, School of Agricultural Sciences and Wildlife Management, Department of Animal Science, P. O Box 60293, Livingstone, Zambia
*Corresponding author: Chisowa DM, Southern University, School of Agricultural Sciences and Wildlife Management, Department of Animal Science, P. O Box 60293, Livingstone, Zambia
Submission: September 22, 2021;Published: October 26, 2021

ISSN: 2770-6729Volume 2 - Issue 1
Abstract
A study was conducted to evaluate the levels of tannins in legume grains (LG) [(soybean (Glycine max), pigeon pea (Cajanus cajan) and Cowpea (Vigna unguiculata)] their effects on trypsin activity and performance of rabbits (Oryctolagus cunniculus). The Flemish Giant (FG) breed was used in the study. Eighteen rabbits weaned at six (6) weeks of age were used out of which six (6) were assigned to ration 1(containing 31.6% soybean), six (6) to ration 2 (containing 72% cowpea) and another six (6) to ration 3 (containing 70.18% pigeon pea). The three (3) (LG) used [soybean (SB), pigeon pea (PP) and cowpea (CoP)] were roasted to reduce trypsin inhibition (TI) before feeds were compounded. The effectiveness of the roasting in reducing TI in the legume grains: SB, PP and CoP were 93%, 52.48% and 82.24% respectively. The effectiveness of roasting in reducing TI in the SB, PP and CoP differed significantly (p˂0.05). The raw legume grains (RaLG), roasted legume grains (RoLG) and the three (3) rations (1, 2 and 3) were analysed for TI level. All the three (3) RaLG differed significantly (p˂0.05) in TI (87.94% for raw SB, 72.73% for raw CoP and 69.27% for PP. Similarly, the three (3) RoLG differed significantly (p˂0.05) in TI (5.87% for roasted SB, 34.56% for roasted CP and 12.30% for roasted PP). Rations 1, 2 and 3 were found to have 1.72%, 20.40% and 8.26% TI, respectively and these values differed significantly (p˂0.05). RaLG, RoLG and the three (3) rations (1, 2 and 3) were analysed for tannin content (TC). The RaLG differed significantly (p˂0.05) in TC (0.33% for SB, 0.40% for PP and 1.13% for CoP). Similarly, RoLG differed significantly (p˂0.05) in TC (0.72% for SB, 0.78% PP and 1.79% for CoP). Rations 1, 2 and 3 were found to possess 0.13%, 0.15% and 0.57% TC respectively.
Keywords:Pathological; Hypertrophy; Tannin; Legume grains; Rabbit; Soybean; Cowpea; Pigeon pea
Introduction
Grain legumes (except for soybean) are potential sources of energy and amino acids for rabbits, but their use is still limited because of uncertainty about the amount and effect of any anti-nutritional factors (ANF) that they may contain. The most commonly found ANFs in legumes are protease (trypsin and chymotrypsin) inhibitors, tannins, lectins, amylase inhibitors, glycosides, phytate and alkaloids. The improvement of the nutritive value of legume grains was related to increases in the metabolizable energy (ME) values (94.57 to 220.12kcal/kg liveweight) and digestibility (70.11 to 77.30% on DM basis) of the proteins of the grain legumes [1,2]. An increasing human demand for protein in developing countries and a relatively high cost of imported ingredients has turned the attention of animal nutritionists to the exploitation of non-conventional ingredients and by-products which these regions have in abundance. Other grain legumes are potential substitutes for soybean meal because of the similarity of their amino acid profiles. Other legume grains could totally replace soybean meal without adversely affecting weight gain provided suitable processing methods are established. The ingredients included will vary between countries and between districts within countries depending on the potential availability of the ingredients for particular livestock enterprises. Traditionally, maize and soybean are used as primary ingredients in most countries. However, the potential of other legume grains relative to maize and soybean is not known. McNitt [3] reported that many problems still remain unsolved in rabbit meat production and less information is available on optimal feeding, breeding, disease prevention and management systems.
Makinde et al. [4], reported that higher (100 %) raw cowpea feeding in pigs resulted in pathophysiological changes in gut morphology leading to haemorrhage in the gut, impaired absorption of nutrients and sometimes death. These authors further observed that anti-nutritional factors in raw cowpeas could account for changes in gut morphology and faecal parameters. The observed decrease in growth of pigs was due to reduced digestive and absorptive capacities as a result of intestinal mucosal changes [4]. It is postulated that some diseases such as enteritis in rabbits are initiated by dietary components, such as lectins, tannins or allergens, which induce diarrhoea and consequently create the favourable environment in the caecum for secondary opportunistic bacterial involvement [5]. Nutritional factors can affect animals indirectly in a number of ways by, for instance creating conditions which favour commensalistic organisms e.g., Clostridium perfringens type D, the cause of pulpy kidney in sheep. Nutritional stress can also induce pathological lesions of the gastro-intestinal tract (GIT) which are ideal sites for bacterial infiltration and colonization [5].
Cowpeas varied in their nutritive value for livestock because of variation in tannin content depending on variety and environmental growing conditions [6]. Le Guen et al. [7] reported that there existed a negative correlation between concentration of cowpea anti-nutritional factors and cowpea protein digestibility in piglets. Thus, in addition to reducing percentage composition of cowpeas, processing of cowpeas was necessary if they were to be recommended as a suitable alternative to soybean in stock feed [4]. In few cases, cowpea haulms have been fed to rabbits or chickens and pigs, with higher rates of gain. Little is known about the potential of cowpeas as a sole source of protein in rabbits [8].
Effects of anti-nutritional factors in rabbit nutrition
Many tropical plants (forages and grains) contain substances that can be toxic and if consumed in large amounts can lead to severe distress and even death. Some legume grains contain substances that render proteins unavailable to the animal for utilisation. These substances inhibit enzyme activity and eventually lower the digestibility of the feed.
Non-ruminants especially rabbits were observed to be more sensitive to anti-nutritional factors contained in legume seeds [9]. Rabbits have the lowest toxic tolerance (0.3mg/kg body weight) than any domestic livestock species [5]. McLeod [5] reported that many tropical plants used as feed for rabbits contained substances that could be toxic if consumed in large amounts and could lead to severe distress and even death. Leaves of these plants were generally high in protein and one such plant that has been widely known as a rabbit feed is Leucaena leucocephala, which contains the toxic amino acid mimosine. Shelton et al. [10] found that some varieties of Leucaena were high in mimosine and could lead to hair loss (alopecia) and possibly impaired reproduction if fed at more than 10-20% of the total dry matter intake. Fortunately, mimosine is partially deactivated if the leucaena is dried before feeding. It is also increasingly possible to obtain low mimosine varieties of Leucaena.
Barry et al. [11] reported that multipurpose legume trees with detectable amounts of condensed tannins such as Caliandra calothyrsus (17.9gkg-1DM), Acacia angustissima (10.3gkg-1 DM) and Flemingia macrophylla (3.4gkg-1 DM) were poorly degraded in the gastrointestinal tract compared to those with undetectable tannins. The presence of condensed tannins could lower their potential as feeds. The efficiency of feed utilization (EFU) was reduced if dietary tannin concentration exceeded 2.6g/kg [12]. At a level greater than 1.5% dry matter condensed tannins fed to monogastric animals caused endogenous protein wastage and inhibition of gastrointestinal enzyme activity [11]. Tannins found in legume grains had the potential to decrease metabolizable energy (ME) by 1.34MJ/kg DM [13]. Further Grosjean et al. [14] reported that ileal protein and amino acid digestibility’s decreased linearly with increased trypsin inhibition activity (TIAP) per unit of crude protein. Ileal digestibility values (%) decreased by -0.1975, -0.1617, -0.2171, -0.2630, -0.2629 and –0.3536 per unit of TIAP, respectively, for crude protein and lysine, threonine, methionine, cystine and tryptophan [14].
Legume grains were characterised by high lysine content (7.3%CP in peas) and a relative deficiency in sulphur-amino acids and tryptophan [14]. Protein digestibility in cowpeas was slightly lower than in soybean meal especially for pigs (0.74 in cowpeas and 0.80 in soybean meal) and for young animals and appeared variable both between and within species. Cowpea trypsin inhibitor activity (TIA) decreased enzyme activity. The higher the trypsin inhibitor activity the more resistant the cowpea protein was to digestion [15]. This lower digestibility could partly be explained by the presence in some species of cultivars of anti-nutritional factors leading to low accessibility of legume seed protein to digestive enzymes [16].
Most ANF exerted their negative effect through interference with normal digestive functions. Heat caused denaturation of proteinaceous inhibitors. Heating was an appropriate method of decreasing the activity of lectins, and also that of trypsin and chymotrypsin inhibitors [17]. Several different heat treatments can be applied to reduce the lectin activity (LA) and ‘heat-labile’ trypsin inhibitor activity (TIA) which generally result in improving the nutritional value of the grain legumes. With prolonged or elevated heating, basic amino acids, such as lysine, underwent a Maillard reaction making them less available for growth [18]. It is, therefore, important to find the exact conditions of heating which maximize the improvement of the nutritive value of the legumes.
Effects of toxic components in commercial rabbit feeds
Commercially produced rabbit feeds, and the individual feed components can contain ‘toxic’ substances which, depending on their concentration may adversely affect the health of rabbits. Nutritional factors affected rabbits indirectly in a number of ways; for instance, by creating conditions which favoured commensalistic organisms, e.g., Pasteurella and Coccidia species which caused Pasteurellosis and Coccidiosis respectively in rabbits [5]. It was postulated that mucoid enteritis was initiated by a dietary component, either a lectin or allergen, which induced the diarrhoea and consequently created the favourable environment in the caecum for secondary opportunistic bacterial involvement [5].
Lectins, which are common in most plant materials used for rabbit pellet production, are capable of eliciting a response in the intestinal tract of rats similar to those observed in rabbit mucoid enteritis. Plant lectins have been shown to stimulate mucous secretions, proliferation of coliform organisms, pathological and histological changes in the GIT epithelial wall of rats, and to cause the microvilli of rats to become fragmented and disrupted. Lectins were responsible for initiating epithelial cell degradation in rabbitgastro- intestinal tract [19].
Statement of the problem
Legume grains are the basis for protein supply in commercial
feeds. However, though they may be rich in protein some contain
anti-nutritional factors which confer negative effects on the
performance and health of livestock. The effects and levels of these
factors such as tannins have not been satisfactorily evaluated.
a. To evaluate the levels of tannins in soybean, pigeon pea and
cowpea grains.
b. To measure the degree of trypsin inhibition in soybean, pigeon
pea and cowpea samples
Materials and Method
Research site
The research was carried out at the Rabbit Research Unit of the Department of Animal Science at the main farm of Bunda College of Agriculture in Lilongwe. Bunda College of Agriculture lies at 1100m above sea level, 14o 11’ S latitude and 33o 46’ E longitude [20]. Bunda has a seasonally wet tropical climate with temperatures modified by its altitude [21]. The mean annual rainfall is approximately 1100mm. Soils vary from Sandy Clay Loam to Sandy Loam with medium fertility (means of 0.1% N and 16.03μg P/g) [22]. During the feeding trial, the average relative humidity was 55.1% while minimum and maximum temperatures were 12.9 oC and 30.2 oC, respectively.
Methodology
Processing of feed ingredients
Table 1:Composition of Rabbit Grower Rations.
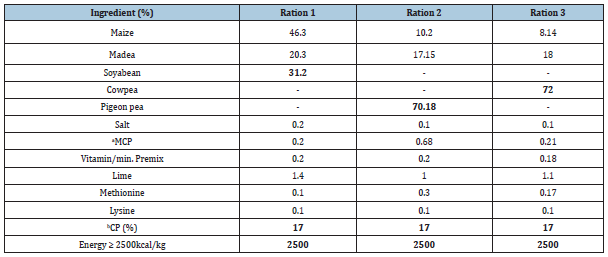
aMCP=Monocalcium phosphate bCP = Crude Protein
The major protein sources in the rations were roasted prior to grinding and mixing to reduce trypsin inhibitor. The trypsin inhibitor in raw and roasted Soybean, Cowpea and Pigeon pea was determined by the laboratory procedure developed by Kalade et al. [23]. The effectiveness of roasting was calculated prior to feed formulation. Results of trypsin inhibitor analysis are shown in Table 1. The ingredients were then ground using a hammer mill and mixed according to the formulae using a feed mixer at Bunda College of Agriculture farm.
Ration formulation
Rations were prepared using the BLP 88 computer programme [24]. The rations were formulated to meet the basic energy and nutrient requirements of growing rabbits [25]. The rations were formulated to contain 17% CP and 2500kcal/kg using the Linear Programme with Bounds (BLP88) computer package [24]. All other ingredients were the same for the rations but only differed in the source of protein.
Determination of tannins
Following high mortality, poor feed intake, low body weight and post-mortem results observed in rabbits offered cowpea ration, the legume grains and the three rations were analysed for tannin content. Tannin content was analysed using the Folin-Ciocalteu assay technique in the Animal Nutrition Research Laboratory at Bunda College. Absorbance for each sample as indicator of tannin concentration was read from a spectrophotometer. Tannic acid levels in micrograms for the three samples were read from the standard curve and the tannin content was calculated as percentage on dry matter basis.
Research design and feeding trial
Rabbits were weighed at the start of the feeding trial. The average weight for the eighteen (18) kits was 0.761kg. The Completely Randomized Design (CRD) was used in the study. The weaners were fed on the test feeds for 7 days so that they could adapt to the new feeds before data collection started. Each rabbit was weighed at the end of the adaptation period, and this was taken as the initial weight for the feeding period of eighty-four (84) days. The three (3) grower rations were replicated twice in the experiment. The rabbits were offered one hundred (100g) grammes of feed once daily during morning hours (07.00 hours) over a 24-hour period and the rejected feed was weighed the following morning (07.00 hours). The difference was taken as the feed consumed by each rabbit per day. Rabbits were weighed individually weekly (every Friday) to obtain weekly body weight from which average daily weight gain and weekly body weight gain were calculated.
Clean water and feed were provided everyday using drinkers and feeders mounted in each cage. Each rabbit was offered seven (7g) grammes of Leucaena leaf meal (LLM) as a source of fibre twice a week. The rabbits were weighed weekly until they reached the targeted average weight of 2.5kg.
Statistical models

Where Yi=observed tannin level in a given ith legume grain type.
μ=Overal mean
Li=effect of the ith legume grain type
b(x)=b is the regression coefficient for initial tannin level used
as a covariate
εi=random error component

Where Yi= observed trypsin inhibition in a given ith legume
grain type.
μ=Overal mean
Ii=effect of the ith legume grain type
b(x)=b is the regression coefficient for initial trypsin inhibition
used as a covariate
εi=random error component
Data collection
Data pertaining to tannin content (%) and trypsin inhibitor (%) in each legume grain type were recorded (Tables 2 & 3).
Table 2:Level of Trypsin Inhibition.
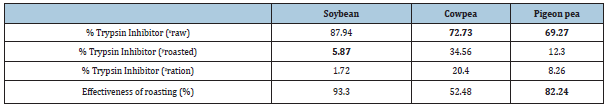
xInhibitor in raw Soybean, Cowpea and Pigeon pea.
yInhibitor in roasted Soybean, Cowpea and Pigeon pea.
zInhibitor in rations (Soybean, Cowpea and Pigeon pea based).
Table 3:Means for Tannin Content (%) in Soybean, Pigeon peas, Cowpeas and Rations 1, 2, 3.
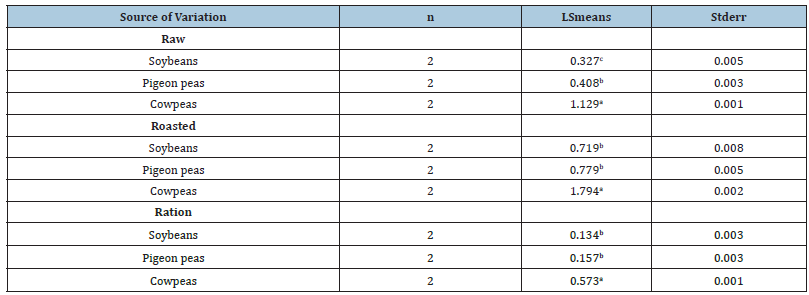
abcMeans with different superscripts are significantly different (p<0.05)
1dcontains 31.2 % soybean CV=1.13 %
2econtains 70.18 % pigeon peas n=number of replications
3fcontains 72.00 % cowpeas
Statistical analysis
Data was analysed using the Statistical Analysis System [25] on the General Linear Model computer. Treatment means were compared using the F-test.
Result
Table 3 below shows the composition of the rations (1, 2 and 3) containing the three (3) legumes (soybean, pigeon pea and cowpea) as sources of protein. The rations contained roasted and ground grain concentrates of legumes. Inclusion levels were based on the standard species nutrient requirements.
Six weaners were lost during the feeding trial, one of which was from a soybean-based ration while the rest were from a cowpeabased ration. The weaner from a soybean-based ration died of pneumonia. Post-mortem of the weaners from a cowpea-based ration revealed that all of them had hypertrophy of kidneys and pale-cracked livers. In addition, three (3) of these weaners were diagnosed with coccidiosis and pasteurallosis. Most of the rabbits offered cowpea ration had low feed intake, low body weight gain and were emaciated. Coccidiosis is usually associated with rabbits raised under a deep litter system and is not common in rabbits raised under battery cage system of production. This implies that coccidiosis was probably an opportunistic infection since these animals were already in poor condition due to the type of ration. All animals were dosed with Esb3 at the rate of 2g Esb3 30% per kg feed to alleviate the problem of coccidiosis. During the feeding trial mortality was at 11.11%.
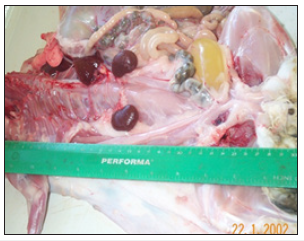
The highest post-weaning mortality was observed in rabbits fed cowpea-based ration. Rabbits fed cowpea-based ration were found to have pale, pathologically enlarged kidneys during postmortem and carcass analysis (Figures 1 & 2). Rabbits fed cowpeabased ration were also found with haemorrhagic intestines and pale livers during post-mortem.
Figure 1:Pathologically enlarged kidneys (k) from a rabbit fed cowpea.
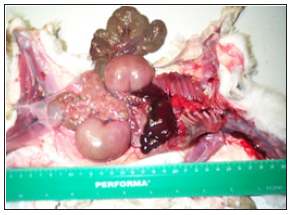
Figure 2:Normal kidneys (k) from a rabbit fed soybean/pigeon pea.
Discussion
Tannins are naturally occurring plant polyphenols that can have a large influence on the nutritive value of legume grains and forage legumes. Tannins are defined as water-soluble polymeric phenolics that precipitate proteins. Tannins exist in mixtures with many other classes of plant phenolic compounds such as lectins [27]. Tannins may reduce intake by decreasing palatability or by negatively affecting digestion. Tannins were reported by Waghorn et al. [28] to lower protein degradation. In the present study the negative effect of tannins on growth rate was caused by a combination of reduced feed intake and low digestibility of proteins. Feed efficiency was highly influenced by feed type.
Results observed in this study support results by Filippich [29] who reported that the major lesions associated with tannin poisoning were; haemorrhagic gastroenteritis, necrosis of the liver and kidney damage with proximal tubular necrosis resulting in death. The poor weight gains observed in rabbits offered cowpea ration can be explained in terms of anti-nutritional factors (ANFs).
Rabbits fed cowpea ration were found with pathophysiological changes in the gastrointestinal tract (GIT), pale and enlarged kidneys (hypertrophy of kidneys), haemorrhagic intestines and pale livers. The average weight of normal kidneys from rabbits fed soybean and pigeon pea was 5.24g, while pathologically enlarged kidneys from rabbits fed cowpeas had an average weight of 20.61g. The average length and width of normal kidneys from rabbits fed soybean and pigeon pea were 3.10cm and 1.00cm, respectively. Pathologically enlarged kidneys measured 6.5cm in length and 2.00cm in width. Three of the five rabbits from cowpea ration were diagnosed with coccidia and Pasteurella protozoa in the GIT and liver. All the five rabbits from cowpea ration which later died gradually deteriorated in health especially from week six of the feeding trial. Observations showed reduced feed intake and lost body weight before death. Hypertrophy of kidneys and pale livers were evident in all rabbits fed cowpea during carcass analysis.
Tannin toxicity in rabbits
Barry et al. [11] reported that the quantity of tannins (60-150g/ kg DM) found in most legume grains is too high for rabbit nutrition. The rabbit is the most sensitive domestic animal to toxicity. While chickens can tolerate up to 2.6 g/kg tannin content, young rabbits can tolerate toxic levels only up to 0.3mg/kg body weight, one of the lowest toxic levels of any species studied [19]. The concentrationof tannins detected in the ration containing cowpea (0.55mg/kg or 0.55%) was high enough to cause pathophysiological changes observed in this study.
Results observed in this study support results reported by Filippich [29] who reported that the major lesions associated with tannin poisoning were; haemorrhagic gastroenteritis, necrosis of the liver and kidney damage with proximal tubular necrosis resulting in death.
The difference in effectiveness among the three (3) rations was manifested in the levels of trypsin inhibitor and tannins in the respective legume grains used as sources of proteins. Heat treatment was least effective in reducing trypsin inhibitor in cowpea and most effective in soybean, while it was satisfactorily effective in pigeon pea. Roasted soybean had the lowest trypsin inhibitor (5.87%), roasted pigeon pea had an intermediate value (20.40%). Cowpea had the highest trypsin inhibitor (34.56%). This implies that soybean had the highest (94.13%) trypsin activity, pigeon pea had intermediate (79.60%) trypsin activity and cowpea had the lowest (65.44%) trypsin activity. Consequently, rabbits fed soybean had the highest weight gain because the proteins were easily degradable.
Tannin levels in legume grains
The results of tannin content of raw, roasted and rations of the three (3) legume grains (Soybean, Cowpea and Pigeon pea) are shown in Table 2. Cowpea had significantly (p<0.05) higher tannin content in all the three (3) samples (raw, roasted and rations). The analysis revealed that tannin content was higher in roasted samples than in raw samples for all the three legume grains.
Carcass quality
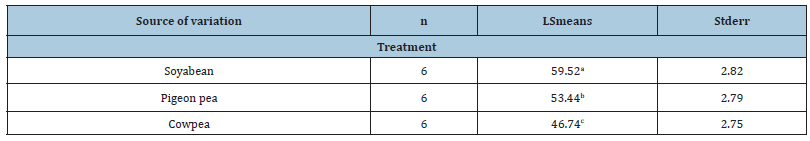
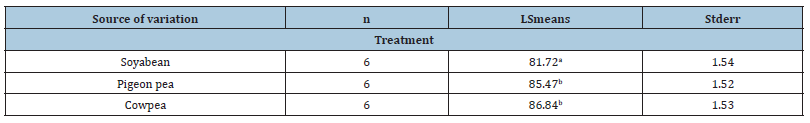
In most livestock, meat quality is affected by breed, feed type and weight at slaughter. In the present study there were little differences in most carcass parameters among the three rabbit breeds used. The differences between breeds or live-weight groups in meat quality tended to be small, which implies a certain constancy in rabbit meat. Results of this study support results obtained by Hernandez et al. [30]. Most carcass traits used for carcass quality evaluation did not differ significantly except for fat percentage and dressing-out percentage. Total fat was observed to vary with breed, sex and feed type. With regard to feed type, fat content was significantly (p<0.001) higher for rabbits fed soyabean than for rabbits fed either pigeon peas or cowpea (Tables 4 & 5).
Table 4:Effect of Treatment on Dressing-out (%) of Rabbits.
abcMeans with different superscripts are significantly different (p<0.05).
Table 5:Effect of Treatment on Lean Proportion (%) of Rabbits.
abcMeans with different superscripts are significantly different (p<0.05).
Dressing-out percentage is an important economic variable in the meat market and the commercial criterion generally used in rabbits is to consider a slaughter yield between 56 % and 58 % from chilled carcass [30]. Results of this study support results reported by Gomez et al. [31] and Pla et al. [32] who observed dressing-out percentages ranging from 55.56% to 59.72% for growing rabbits.
Conclusion
This study has revealed that soybean was lower in tannin content than either pigeon pea or cowpea. At the levels of inclusion used in this study. The effectiveness of roasting to reduce trypsin inhibitor differed among the three legume grains. The level of effectiveness was highest in soybean (93.30%), lowest in cowpea (52.48%) with pigeon pea showing intermediate level (82.24%). The discrepancy in response to same degree of heat treatment by the three legume grains suggests that trypsin inhibitor cannot be equally reduced by the same level of heating. This demonstrates a difference in the nature of the kernels from the three types of legume grains. At a given temperature soybean and pigeon pea respond well to heat treatment (roasting) as a means of reducing trypsin inhibitor.
Rabbits fed cowpea ration were found with pathophysiological changes in the gastrointestinal tract (GIT), pale and enlarged kidneys (hypertrophy of kidneys), haemorrhagic intestines and pale livers. At the level of inclusion and processing used in this study cowpea is not safe for use in rabbit rations. Consequently, much higher heating and lower inclusion levels of cowpea should be considered to mitigate levels of anti-nutritional factors. There is need to investigate safe inclusion levels for cowpea in order to avoid the observed toxicity. It is therefore suggested that in addition to reducing percentage composition (inclusion level) of cowpeas, a more effective method of processing cowpeas is necessary if they are to be recommended as a suitable source of proteins in rabbit rations. There is also needed to study the anatomy of the cowpea grain in order to employ a more effective method of processing.
The results of this study suggest that the variety of cowpea used in these experiments is not suitable as a sole source of protein in rabbit rations. The discrepancy in response to same degree of heat treatment by the three legume grains suggests that trypsin inhibitor cannot be equally reduced by the same level of heating. Consequently, a much higher level of heating is required in cowpea. Even though tannin content in cowpea was observed to exist beyond tolerable levels for rabbits, other toxic compounds found in legume grains could have played a role. There is need for further investigation to establish the impact of individual toxins found in legume grains. Inclusion level for cowpea lower than that used in this study should be investigated to reduce toxicity observed in this study.
Data Availabilit
Readers can access the data used in the conclusions for this article by contacting the corresponding author through the following contact details: Email: mcmchisowa@yahoo.com.sg
Conflict of Interest
There is no conflict of interest regarding the publication of this article.
Funding Statement
This work was supported by the Southern African Centre for Cooperation in Agricultural Research (SACCAR) and Deutsche Gesellschaft for Technische Zusammenarbeit (GTZ).
Acknowledgment
I wish to thank Dr. T. Mujahid for the assistance rendered during analysis for tannin and Mrs. D. Salifu for her advice on statistical procedures throughout the study period. I appreciate the assistance offered by Dr. M. W. Mfitilodze and Mr. W. W. D. Mvula in conducting post-mortem on the rabbit carcasses. I would also want to thank Mr. C. Kayange, Mr. E. Nyali and Mr. F. Kasenda all of Bunda College of Agriculture for their contribution in laboratory analysis of samples and statistical data analysis.
References
- Dourandish S, Mousavizade SM, Ezatpour HR, Ebrahimi GR (2017) Microstructure, mechanical properties and failure behavior of protrusion friction stir spot welded 2024 aluminium alloy sheets. Science and Technology of Welding and Joining 23(4): 295-307.
- Gopi S, Manonmani K (2012) Study of friction stir welding parameters in conventional milling machine for 6082-T6 aluminum alloy. Australian Journal of Mechanical Engineering 10(2): 129-140.
- Padhy GK, Wu CS, Gao S (2018) Friction stir based welding and processing technologies - processes, parameters, microstructures, and applications: A review. Journal of Materials Science & Technology 34(1): 1-38.
- Huang Y, Lv Z, Wan L, Shen J, Dos Santos JF (2017) A new method of hybrid friction stir welding assisted by friction surfacing for joining dissimilar Ti/Al alloy. Materials Letters 207: 172-175.
- Rajesh G, Srimuthunath I, Santa Kumar N, Sugandipriya S, Aravindhan V (2018) Characterization of microstructure and hardness of friction stir welded brass plate. Materials Today: Proceedings 5(1): 2721-2725.
- Kamal Jayaraj R, Malarvizhi S, Balasubramanian V (2017) Electrochemical corrosion behavior of stir zone of friction stir welded dissimilar joints of AA6061 aluminum-AZ31B magnesium alloys. Trans Nonferrous Met Soc China 27(10): 2181-2192.
- Abhinand (2017) Numerical simulation of friction stir welding for dissimilar metal welding. Materials Today: Proceedings 4 (10): 11265-11269.
- Xueqi Lv, Song Wu C, Yang C, Padhy GK (2018) Weld microstructure and mechanical properties in ultrasonic enhanced friction stir welding of Al alloy to Mg alloy. Journal of Materials Processing Tech 254: 145-157.
- Kumar R, Singh R, Ahuja IPS, Penna R, Feo L (2018) Weldability of thermoplastic materials for friction stir welding- A state of the art review and future applications. Composites Part B: Engineering 137: 1-15.
- Annand, Kumar Barik B, Tamilmannan K, Sathiya P (2015) Artificial neural network modeling studies to predict the friction welding process parameters of Incoloy 800H joints. Engineering Science and Technology, an International Journal 18(3): 394-407.
- Shaik B, Gowd H, Durga Prasad B, Siddik P (2022) Investigations on microstructures by using friction stir processing. Intelligent Manufacturing and Energy Sustainability, Smart Innovation, Systems and Technologies, pp. 539-548.
- Shaik B, Harinath Gowd G, Durga Prasad B (2019) Parametric investigations on friction stir welding of aluminium alloys. Emerging Trends in Mechanical Engineering, Lecture Notes in Mechanical Engineering, pp. 333-345.
© 2021 Chisowa DM. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






