- Submissions

Full Text
Clinical Research in Animal Science
Effects of Sub-MIC of Rifaximin and Essential Oils on Biofilm Formation and Invasion of Staphylococcus aureus Isolated from Mastitis
Xiangyuan Jiang, Fanxi Guo, Ying Li and Zugong Yu*
Laboratory of Veterinary Pharmacology and Toxicology, College of Veterinary Medicine, Nanjing Agricultural University, China
*Corresponding author: Zugong Yu, Laboratory of Veterinary Pharmacology and Toxicology, College of Veterinary Medicine, Nanjing Agricultural University, Nanjing 210095, China
Submission: July 02, 2021;Published: July 15, 2021

ISSN: 2770-6729Volume 1 - Issue 4
Abstract
Significance and Impact of the Study
Mastitis can be caused by Staphylococcus aureus, which is hard to be eradicated, especially when it formed biofilm. This situation has made searching for novel antimicrobial agents urgent. Present study demonstrates the anti-bacteria and anti-biofilm inhibitory activity of essential oils on S. aureus alone and combined with rifaximin. Combinations of rifaximin and mint oil can not only inhibit S. aureus from growing on abiotic material, but also restrain its invasion into cells. Results of the present study gave an insight into the promising alternatives for killing S. aureus and treating mastitis.
Abstract
This study intended to explore whether rifaximin and EOs (mint oil (MEO), eucalyptus oil (EEO), and EO of Houttuynia cordata (HEO)) alone and combined can inhibit the biofilm formation and invasion ability of S. aureus. Firstly, minimal inhibitory concentration (MIC) of 9 strains were investigated. Otherwise, checkerboard microdilution assays were used to measure FICI of rifaximin and EOs of S. aureus- 2. Lastly, the influence of the sub-MIC of EOs alone and combined with rifaximin on the biofilm formation and invasion of S. aureus were tested. MEO exhibited the greatest efficacy against planktonic cells. The results demonstrated that combination of rifaximin and MEO was additive, and the others showed indifferent. Almost all EOs alone and combined with rifaximin effectively reduced the S. aureus biofilm formation to some extent. Meanwhile, combination of rifaximin and MEO showed the strong inhibitory effect on invasion, and 0.5MIC rifaximin +0.125MIC MEO can inhibit bacteria from invasion. These results suggested rifaximin alone and combined with EOs, in particular MEO, can be the alternative therapies for bovine mastitis.
Keywords:Nanomaterials; Drug delivery; Veterinary medicine
Introduction
Bovine mastitis is the inflammation of the bovine mammary glands, leading to numerous economic losses, such as reduction of the milk production, waste of substandard milk, etc [1]. Bacteria are major causes of mastitis, of which Staphylococcus aureus (S. aureus) is an important one [2]. The most common treatment of mastitis is antibiotic therapy, however, cure rates for S. aureus mastitis are variable, ranging from 4 to 92% [3]. There are studies showed that an important reason for reduced therapy success rates is resistance of S. aureus [4]. The increasing prevalence of antibiotic resistance of S. aureus may contribute to biofilm-forming abilities [5].
Biofilm of S. aureus is a complex microbial community embedded in a self-produced extracellular matrix on abiotic surfaces or animal tissues, such as bovine utter [5-7]. The adhesion and invasion ability of S. aureus may be one of the foundations of biofilm establishment [8]. The matrix can act as a protective layer for bacteria, allowing bacteria to be more resistant to antibiotics, eventually making antibiotics treatment low efficiency [5,9,10].
Current situation has made search for alternative strategies for combating biofilm necessary. Rifaximin is intended for prevention and treatment for mastitis during the dry period, but there were limited articles about its influence on biofilm. EOs, which are natural antimicrobials, may be potential therapy to combat biofilm [11]. Moreover, EOs can also inhibit S. aureus from invasion, such as citrus-derived oil [12]. Otherwise, combination therapy can be a promising alternative in that it may extend spectrum coverage, prevent resistant mutants from emergence and gain synergy between drugs [13]. What’s more, hydrophobic feature of EOs may render cells more permeable to the uptake of antibiotics [14]. Which is to say, the combinations of EOs and traditional antibiotics may be available treatment approaches.
Based on above, trails had been carried out to discover whether rifaximin and EOs alone and combined can inhibit biofilmformation, and co-culture model was established to figure out the effect of the rifaximin and MEO on adhesion and invasion of S. aureus into MAC-T.
Result and Discussion
Antibacterial activity of rifaximin and EOs alone and combined
The MICs of antibiotics and EOs against S. aureus in suspension were shown in Table 1. Some MIC were over 5%, HEO at the concentration of 2.5% failed to kill most of the stains of our experiment, while EEO was able to kill most of the bacteria at the same concentration, as for MEO, the MIC of that needed to eradicate cells in suspension was below or equal to 2.5%. Which is to say, MEO showed the highest inhibitory effects on planktonic cells of all strains tested.
Table 1: MIC of essential oils.
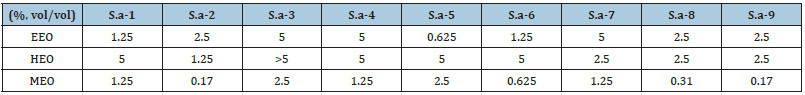
S.a represents for S.aureus.
As Table 2 showed, rifaximin in combination with MEO showed an additive effect (0.5< FICI≤1), the rest two combinations had an indifferent effect (1< FICI≤2). There were reports indicating that the hydrophobicity of EOs may render cell membrane more permeable to the uptake of antibiotics, and components of essential oils as thymol and carvacrol can have same effect, too [15,16]. But the naturally derived antimicrobials have more biochemical and structural diversity than common drugs [10].
Table 2: The FICI of Rifaximin and EOs against S. aureus.
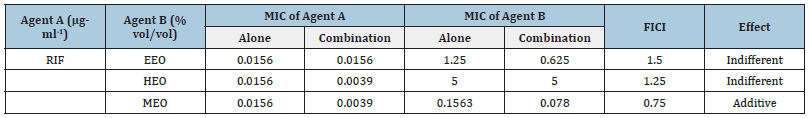
Anti-biofilm activity of rifaximin and EOs alone and combined
Figure 1: Effects of EOs at sub-MIC on S.aureus biofilm formation. The biofilms were stained with crystal violet and the optical density was determined with a multi-detection microplate reader of OD595nm. Data shown are representative three independent experiments and are expressed as mean ±SD. A. EEO treatment group. B. HEO treatment group. C. MEO treatment group. *Represent statistical significance (P≤0.05), **Represent extreme significance (P≤0.01).
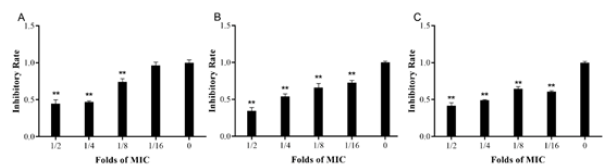
The influence of sub-MIC of different kinds of drugs on the biofilm formation of S. aureus-2 was determined through CV staining. Results were shown in Figure 1. When treated with sub- MIC of HEO and EEO, inhibition was stronger with the concentration of drugs increasing. HEO at all concentrations exhibited an extreme significant inhibitory effect on biofilm formation compared to the control (P≤0.01). The biofilm treated with EEO at concentration of 0.063MIC had no difference from the control (P>0.05), and other concentrations were different from the control (P≤0.05). There was extreme significance between treatment group of MEO with the control group (P≤0.01). But treatment of 0.125MIC of MEO had stronger inhibition effect than adjacent group, with no difference showed.
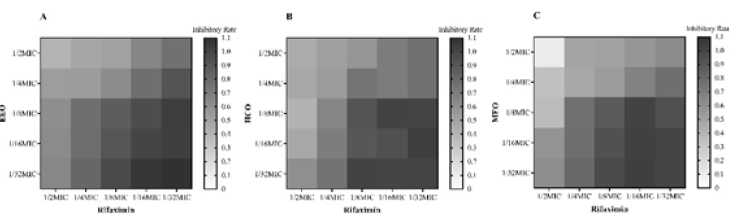
It’s been studied that whether the treatment of established biofilms with combinations of rifaximin and EOs would reduce biofilm biomass more than treatment with EOs alone. As the Figure 2 showed, the combination of 0.5MIC rifaximin+0.5MIC MEO can inhibit the biofilm-forming most effectively, with inhibitory rate of 90%, the combination of 0.5MIC rifaximin+0.5MIC EEO reduced biofilm biomass by 55%, and the combination of 0.5MIC rifaximin+0.5MIC HEO gave a 60% reduction. The combination of drugs whose concentrations are both 0.063MIC shows no difference with the control group.
Figure 2: Effects of rifaximin and MEO at sub-MIC on S.aureus biofilm formation. The biofilms were stained with crystal violet and the optical density was determined with a multi-detection microplate reader of OD595nm. Data shown are representative three independent experiments and are expressed as mean ±SD. A. EEO treatment group. B. HEO treatment group. C. MEO treatment group.
EOs possess antimicrobial and anti-biofilm activity with the potential for use as therapeutic agents [17]. MEO, which belonged mint family, is rich in cavracrol and thymol have a high efficacy against pathogens bacteria [18]. EEO can inhibit biofilm of Staphylococcus epidermidis, E. coli, and Salmonella paratyphi, is a promising agent to be applied to combat biofilm [19]. Extracts of H. cordata showed to be anti-bacterial and anti-biofilm, but the effect of EOs on biofilm are not studied yet [20-22].
Combination of rifaximin and EOs showed stronger antibiofilm ability than drugs using alone at certain concentrations. However, there are little article involved the combined effect of rifamycins and EOs. Rifampin, the analog of rifaximin, is effective alone or in combination with other antibiotics against the bacteria forming biofilms [23]. Combination can be useful application, since there may be a great chance that bacteria seldom produce monotherapies [14,24]. It’s showed that antibiotics of the cell-wall active class can be combined with rifampin [17]. What’s more, EOs may be able to penetrate on the cell wall of Gram-positive bacteria, in that cell structure of gram-positive bacteria and hydrophobic features of Eos [17,25]. The reports showed that EOs combined with its composition may be more effective than using alone, S. aureus showed malformed cell surface or broken cells with pores formation [26]. The combination of rifaximin and MEO was more effective may be related to the composition, such as mint family, which is rich in cavracrol and thymol [18].
Cytotoxicity of rifaximin and MEO on MAC-T cells
The results of drugs used on this study on MAC-T cells after 3h and 24h were showed in Figure 1. There was no significant cytotoxicity (P>0.05) observed between the group treated with rifaximin and gentamicin and the control group at 3h or 24h. The MEO at 0.125MIC was not significantly different (P>0.05) with the control, however, at the higher concentrations (0.25MIC), extreme significance was observed (P<0.01) at 3h, and significant difference was observed (P<0.05) at 24h. Above all, 0.5MIC RIF and 0.125MIC MEO were chosen for further study.
Effect of rifaximin and MEO on S. aureus invasion into MAC-T
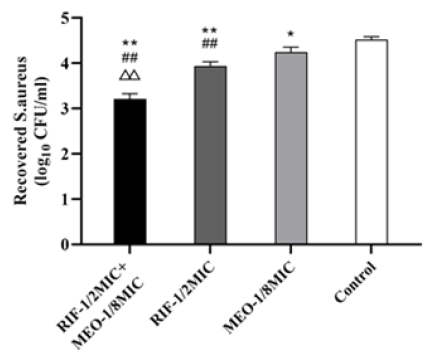
0.5 MIC Rifaximin+0.125 MIC MEO, the non-toxic group, was chosen for invasion assay. The results were shown in Figure 3, showing rifaximin and MEO alone and combined has an effect on bacteria invasion. Invasion of S. aureus into MAC-T cells were reduced in 0.125 MIC MEO and 0.5 MIC Rifaximin groups, respectively, which is different (P≤0.05) and significantly different (P≤0.01) compared with the control group. The result showed that treated group of 0.5 MIC Rifaximin+0.125 MIC MEO was significantly different (P≤0.01) compared with the control group, 0.5 MIC Rifaximin group and 0.125 MIC MEO group. The effect of drugs alone and combined on invasion confirmed that rifaximin and MEO can inhibit S. aureus from attaching to biotic and abiotic material, and the effect of drugs combined was more effective than alone. There were no reports about MEO’s effect on invasion to our knowledge, not to mention the articles about combination of antibiotics and EOs. Intramammary bacterial adherence is an important factor of biofilm formation, at the same time, the biofilm formed appears to enhance binding of S. aureus to epithelial cells [27]. Mammary epithelial cells are vital for surveillance of mammary tissue by recruiting immune cell and recognizing bacterial during infection [28]. Invasion of S. aureus into bovine mammary epithelial cells was related to surface-associated proteins, further study is required to find out whether MEO effecting biofilm-related genes and surfaceassociated proteins [29].
Figure 3:Effect of rifaximin and MEO on S.aureus invasion into MAC-T. A co-culture group with no drug added was used a control. *Represent statistical significance with control group (P≤0.05), **Represent extreme significance (P≤0.01); #Represent statistical significance with MEO group (P≤0.05), ##Represent extreme significance (P≤0.01); △Represent statistical significance with RIF group (P≤0.05), △△Represent extreme significance (P≤0.01).
Material and Methods
Bacterial isolates and culture conditions
Nine Staphylococcus aureus (S. aureus) isolates were obtained from milk acquired from bovine suffered from bovine mastitis in a dairy farm at Pingdingshan, Henan, China. S. aureus-2 was choosing for further study for its strong biofilm-forming ability. The isolate was cultured in TSB at 37 ℃ for 8-10h (mid-log phase) for further study.
Mammalian cell culture
Bovine mammary alveolar cell line (MAC-T, BNCC338264, BNBIO, Beijing, China) was used for invasion assays. MAC-T cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Hyclone, Logan, UT, USA) supplemented with 10% (vol/vol) fetal bovine serum (FBS; Gibco, Invitrogen, Carlsbad, USA) at an incubator with 5% (vol/vol) CO2.
Antimicrobial agents and essential oils
Rifaximin was purchased from National Institutes for Food and Drug Control (Beijing, China). Three EOs, MEO, HEO and EEO were buying from Huayuan Perfume Company. Stock solutions (1280μg·ml-1) of rifaximin were prepared in dimethyl sulfoxide (DMSO), sterile filtered (0.22-μm pore diameter) and stored at -20 ℃ for 2 weeks. Stock solutions at concentration of 50% EOs were solubilized with equal amount of ethanol plus Tween-80 and dissolved in TSB to produce solution of 10% used for further study.
MIC
MIC for EOs and antibiotics was determined by the microdilution methods according to Clinical and Laboratory Standards Institute [30]. Briefly, culture in mid-log phase was diluted to 106CFU·ml-1 and 100μl suspension was then added into wells supplemented with 100μl EOs solution which is serially two-fold diluted. After incubation overnight, MIC was determined as the lowest concentration preventing growth.
FIC assay by the checkerboard method
Combination susceptibility of rifaximin and EOs was tested by the checkerboard micro-dilution method. Rifaximin and EOs were serial two-fold dilutions in two 96-well microtiter plates with final concentration ranging from 16 to 0.5-fold of the MIC. Then, 50μl of rifaximin and 50μl of EOs serial dilutions were added horizontally and vertically into the plates, 100μl of inoculates (106CFU·ml-1) were added into all wells, and incubated at 37 ℃ for 24h. The formulas used to calculate the FICI are as followed: FICI=FICIA+ FICIB=MICA Combined/MICA alone + MICB Combined/MICB alone. The result was used to explain the effects of combination susceptibility of the drugs: synergy (FICI≤0.5), interaction (0.5< FICI≤1), indifference (1< FICI≤2), and antagonism (FICI>2). The tests were performed in three replicates.
Biofilm production at sub-MIC of rifaximin and EOs alone or combined
Biofilm formation in the presence of sub-inhibitory rifaximin and EOs concentrations (0.5MIC-0.063MIC) was performed according to the method previously [31], with modifications. S. aureus culture (100μl, 106 CFU·ml-1) was added to 96-well microtiter plates, and drug solutions were added to attain a final concentration at 0.5MIC to 0.063MIC. TSB broth (100μl) mixed with 100μl of S. aureus culture was used as positive control, and TSB broth (200μl was used as negative control. The microplates were incubated at 37 ℃ for 48h, and biofilm production was dyeing with CV and taking readings at OD595nm. All tests were performed in three replicates.
Cytotoxicity of rifaximin and MEO alone or combined on MAC - T Cells
The 3 - (4,5- dimethylthiazol-2-yl) - 2,5-diphenyltetra zolium bromide (MTT) assay methods were applied for cytotoxicity of drugs on MAC-T. Epithelial cells (104 cells per well) were added to a 96-well plate at 37 ℃, 5% CO2 overnight. The medium was removed, and cells were washed 3 times. The plate was added with 200μl of DMEM supplemented with 10% FBS, rifaximin at 0.5MIC and MEO at 0.5MIC, 0.25MIC, or 0.125MIC alone or combined, and gentamicin at 100μg·ml-1, and incubated for 3h at 37 ℃, 5% CO2. Non-treated cells and medium were positive and negative controls. Medium was removed again, and the plate was washed 3 times with DMEM before 200μl of DMEM supplemented with 20μl of 5mg·ml-1 of MTT was added. The cells were incubated for 4h, afterward, medium was removed and 150μl of DMSO was added to each well. OD490nm of the plate was tested after shaking for 10 to 15 min. 100% DMSO was used as blank. The tests were performed in triplicate.
Effect of rifaximin and MEO on S. aureus invasion into MAC-T
To quantify the adherent and invaded bacteria, an adhesion assay was performed according to Silva et al (Silva et al., 2014), with modifications. Briefly, S. aureus was grown in 5ml TSB overnight at 37 ℃. Bacteria were centrifuged (5min, 5,000r·min-1, 4 ℃), washed twice three times with phosphate-buffered saline (PBS, pH 7.4; Solarbio), and resuspended in cell culture medium. Monolayers of MAC-T cells (105 cells per well) were cultured in 24-well plates (Falcon, Corning Inc., NC, USA). were washed three times with PBS and infected by S. aureus at a multiplicity of infection (MOI, the ratio of S. aureus to MAC-T) of 100. At the same time, drugs were added at a final concentration of 0.5 MIC RIF, 0.125 MIC of MEO, and 0.5 MIC RIF+0.125 MIC of MEO. After 3h of invasion, MAC-T monolayers were washed with PBS for three times, non-internalized bacteria were killed by gentamicin (100μg·ml-1) for 2h. Monolayers were washed three times with PBS and lysed with 1ml of 0.25% (wt/ vol) trypsin and 0.1% (vol/vol) Triton X-100 in PBS for 10 min to obtain the intracellular bacteria. Lysates were 10-fold serially diluted and cultured on MH agar at 37 ℃ for 24h. Control wells (without antimicrobials) were performed as above. Each assay was performed with triplicate samples.
Statistical analysis
A significance level of 5% (P>0.05) was used for statistical analyses. All data were presented as mean ± standard deviation. Statistical analysis was performed by using SPSS19.0 (IBM Corp, Armonk, NY, USA) and GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA). One-way analysis of variance (ANOVA) followed by Tukey’s test was used for multiple comparisons.
Funding
This study was supported by the Priority Academic Program Development of Jiangsu Higher Education Institution (PAPD).
Conflict of Interest
No conflict of interest declared.
References
- Budri PE, Silva NCC, Bonsaglia ECR, Fernandes A, Araújo JP, et al. (2015) Effect of essential oils of Syzygium aromaticum and Cinnamomum zeylanicum and their major components on biofilm production in Staphylococcus aureus strains isolated from milk of cows with mastitis. J Dairy Sci 98(9): 5899-5904.
- Lundberg Å, Aspán A, Nyman A, Unnerstad H, Waller K (2014) Associations between bacterial genotype and outcome of bovine clinical Staphylococcus aureus Acta Vet Scand 56(1): 65-66.
- Koen B, Honaker Ryan W, Hobbs Zachary, Richter Manuela, Zaczek Maciej, et al. (2017) Efficacy and safety of a bovine-associated Staphylococcus aureus phage cocktail in a murine model of mastitis. Front Microbiol 8: 2348.
- Barkema HW, Schukken YH, Zadoks RN (2006) Invited review: The role of cow, pathogen, and treatment regimen in the therapeutic success of bovine Staphylococcus aureus Journal of Dairy Science 89(6): 1877-1895.
- Melchior MB, Vaarkamp H, Fink Gremmels J (2006) Biofilms: A role in recurrent mastitis infections? Vet J 171(3): 398-407.
- Flemming H, Wingender J, Szewzyk U, Steinberg P, Rice SA, et al. (2016) Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 14(9): 563-575.
- Sugimoto S, Sato F, Miyakawa R, Chiba A, Onodera S, et al. (2018) Broad impact of extracellular DNA on biofilm formation by clinically isolated Methicillin-resistant and -sensitive strains of Staphylococcus aureus. Scientific Reports 8: 2254.
- Silva VO, Soares LO, Silva Junior A, Mantovani HC, Chang YF, et al. (2014) Biofilm formation on biotic and abiotic surfaces in the presence of antimicrobials by escherichia coli isolates from cases of bovine mastitis. Appl Environ Microbiol 80(19): 6136-6145.
- Boles BR, Horswill AR (2011) Staphylococcal biofilm disassembly. Trends Microbiol 19(9): 449-455.
- Roy R, Tiwari M, Donelli G, Tiwari V (2018) Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 9(1): 522-554.
- Durairajan R, Farisa BS, Prakash N, Ramar M, Subbiah T, et al. (2018) Essential oils from unexplored aromatic plants quench biofilm formation and virulence of Methicillin resistant Staphylococcus aureus. Microb Pathog 122: 162-173.
- Federman C, Joo J, Almario JA, Salaheen S, Biswas D (2016) Citrus-derived oil inhibits Staphylococcus aureus growth and alters its interactions with bovine mammary cells. J Dairy Sci 99(5): 3667-3674.
- Drago L, De Vecchi E, Nicola L, Colombo A, Guerra A, et al. (2004) Activity of levofloxacin and ciprofloxacin in combination with cefepime, ceftazidime, imipenem, piperacillin-tazobactam and amikacin against different Pseudomonas aeruginosa phenotypes and Acinetobacter spp. Chemotherapy 50(4): 202-210.
- Rodrigues FFG, Costa JGM, Coutinho HDM (2009) Synergy effects of the antibiotic’s gentamicin and the essential oil of Croton zehntneri. Phytomedicine 16(11): 1052-1055.
- Helander IM, Alakomi HL, Latva Kala K, Mattila Sandholm T, Pol I, et al. (1998) Characterization of the action of selected essential oil components on Gram-negative bacteria. J Agric Food Chem 46(9): 3590-3595.
- Burt S (2004) Essential oils: their antibacterial properties and potential applications in foods-a review. International Journal of Food Microbiology 94(3): 223-253.
- Vasconcelos NG, Croda J, Simionatto S (2018) Antibacterial mechanisms of cinnamon and its constituents: A review. Microb Pathog 120: 198-203.
- Cosentino S, Tuberoso CI, Pisano B, Satta M, Mascia V, et al. (2010) In-vitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Lett Appl Microbiol 29(2): 130-135.
- Saviuc CM, Drumea V, Olariu L, Chifiriuc MC, Bezirtzoglou E, et al. (2015) Essential oils with microbicidal and antibiofilm activity. Curr Pharm Biotechnol 16(2): 137-151.
- Han EH, Park JH, Kim JY, Jeong HG (2009) Houttuynia cordata water extract suppresses anaphylactic reaction and IgE-mediated allergic response by inhibiting multiple steps of FcepsilonRI signaling in mast cells. Food Chem Toxicol 47(7): 1659-1666.
- Sekita Y, Murakami K, Yumoto H, Mizuguchi H, Amoh T, et al. (2016b) Anti-bacterial and anti-inflammatory effects of ethanol extract from Houttuynia cordata poultice. Biosci Biotechnol Biochem 80(6): 1205-1213.
- Sekita Y, Murakami K, Yumoto H, Amoh T, Natsumi Fujiwara Ogata S, et al. (2016a) Preventive effects of Houttuynia cordata extract for oral infectious diseases. BioMed Res Int 2016: 2581876.
- Scheinfeld N (2016) Why rifampin (rifampicin) is a key component in the antibiotic treatment of hidradenitis suppurativa: a review of rifampin’s effects on bacteria, bacterial biofilms, and the human immune system. Dermatol Online J 22(6): 13030.
- Fischbach MA (2011) Combination therapies for combating antimicrobial resistance. Curr Opin Microbiol 14(5): 519-523.
- Farooq U, Malviya R, Sharma PK (2014) Extraction and characterization of artocarpus integer gum as pharmaceutical excipient. Polim Med 44(2): 69-74.
- Vitanza L, Maccelli A, Marazzato M, Scazzocchio F, Comanducci A, et al. (2019) Satureja montana essential oil and its antimicrobial activity alone or in combination with gentamicin. Microb Pathog 126: 323-331.
- Wei Z, Xiao C, Guo C, Zhang X, Wang Y, et al. (2017) Sodium acetate inhibits Staphylococcus aureus internalization into bovine mammary epithelial cells by inhibiting NF-κB activation. Microb Pathog 107: 116-121.
- Zhang L, Sun L, Wei R, Gao Q, He T, et al. (2017) Intracellular Staphylococcus aureus control by virulent bacteriophages within MAC-T Bovine mammary epithelial cells. Antimicrob Agents Chemother 61(2): e01990-16.
- Lister JL, Horswill Alexander R (2014) Staphylococcus aureus biofilms: recent developments in biofilm dispersal. Front Cell Infect Microbiol 4: 178.
- Clinical and Laboratory Standards Institute (2017) Performance standards for antimicrobial susceptibility testing, CLSI supplement M100. (27th edn), Wayne, Pennsylvania, USA.
- Lopes LAA, dos Santos Rodrigues JB, Magnani M, de Souza EL, de Siqueira Júnior JP (2017) Inhibitory effects of flavonoids on biofilm formation by Staphylococcus aureus that overexpresses efflux protein genes. Microbial Pathogenesis 107: 193-197.
© 2021 Zugong Yu. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






