- Submissions

Full Text
Clinical Research in Animal Science
Effect of refreezing on the plasma membrane integrity, acrosome, viability, morphology and individual motility in bovine sperm
Hincapie JJ1*, Cuellar CJ2, Ross PJ3 and Castillo R4
1Departament of Agricultural Science and Production, Zamorano University, Tegucigalpa, Honduras
2Departament of Agricultural Science and Production, Zamorano University, Tegucigalpa, Honduras,
3Animal Reproduction Department, UC Davis, California, United States
4Departament of Agricultural Science and Production, Zamorano University, Tegucigalpa, Honduras,
*Corresponding author: Hincapie JJ, Departament of Agricultural Science and Production, Zamorano University, Tegucigalpa, Honduras
Submission: February 19, 2021;Published: March 25, 2021

ISSN: 2770-6729Volume 1 - Issue 3
Abstract
The purpose of this study was to evaluate the effect of refreezing of bovine semen, on the individual motility, viability, morphology, Animal Reproduction Laboratory in Zamorano University, Honduras. The semen of a 60-month-old Brahman and integrity of the sperm’s plasma membrane. The research was carried out from November 2018 to September 2019 at the bull, which was collected twice. The electro-ejaculation methodology was applied for the semen collection. Three treatments were applied: Fresh-Semen Diluted (FSD), Frozen-Thawed Semen (FTS) and Frozen-Thawed-Refreezing Semen (FTRS). In fresh semen the ejaculate volume (7mL), color (creamy white), odor (typical of the species), mass motility (>90%), individual sperm motility (>90%), concentration (739 x106/mL) and morphology (>90% normal) were evaluated. Subsequently, the semen was diluted using a TRIS- based diluent, citric acid, sugar, egg yolk, buffers, glycerin, double-distilled water, and the following antibiotics: tylosin, gentamicin, spectinomycin and lincomycin. A sample of the FSD was taken and evaluated for: individual motility, viability, morphology, and Hypo-Osmotic-Swelling-Test (HOST). The remaining diluted semen was packed in 0.5cc straws at a concentration of 30x106 sperm/straw, and frozen at -196 °C in liquid nitrogen. After five days, 20 straws were randomly taken and subjected to the FTS treatment, which consisted of thawing the straws at 38 °C/45 seconds, using water bath and further evaluation while another 20 straws were put through to the FTRS treatment, which consisted of defrosting the straws at 38 °C/45 seconds in a water bath. Subsequently they were left at room temperature for 60 minutes, and then were re-frozen, placing them at 4 °C for four hours (equilibrium) and then subjected to liquid nitrogen vapors for 10 minutes after that they were introduced in liquid nitrogen -196 °C. After 24 hours, six straws were thawed at 38 °C/45 seconds to be re-evaluated. The analyzed variables were individual motility (%), biological quality (individual post-frozen motility per concentration. The results must be greater than 10.5 x 106 sperm to be considered as a suitable sample for artificial insemination); Viability (% alive and dead using the BLOOM technique); Morphology (% normal sperm using Spermac® staining); Integrity of the plasma membrane using the Hypo-Osmotic-Swelling-Test (HOST): percentage (%) of Positive Endosmosis (PE) that corresponds to sperm with different degrees of curl/swelling of the tail and Negative Endosmosis (NE) that corresponds to the sperm without reaction in the tail. For the viability, morphology and membrane integrity tests (HOST test), at least 1200 sperm were counted in each one. In the individual motility and biological quality tests, at least 10 optical fields per test were evaluated. A Completely Randomized Design (CRD) was used with three treatments (FSD, FTS, FTRS) and two repetitions per treatment. The arc-sin function was applied for the conversion of percentage values and an Analysis of Variance (ANOVA) using the General Linear Model (GLM) and separation of means with the Duncan multiple range test, using the Statistical Analysis Systems program (SAS) with a required level of significance of P≤0.05. In semen FSD, FTS and FTRS the percentages of individual motility were 92.5%; 65.42% and 46.25% respectively (P≤0.05); the percentages of live sperm (P≤0.05) were 84.18%; 61.70% and 48.21% respectively and the percentages of normal sperm were 97.6%; 95.97% and 93.46% respectively. Regarding the HOST tests, the values were 82.5%; 50.13% and 31.58% for FSD, FTS and FTRS respectively (P≤0.05). The biological quality was 19.6 and 13.8 x 106 for FSD and FTRS respectively (P≤0.05). A high correlation (P<0.0001) was found between Positive Endosmosis with individual motility, biological quality, normality, and viability. The findings indicate that the freezing, thawing and refreezing process, affect the plasma membrane integrity, resulting in a higher percentage of sperm with Positive Endosmosis the FSD treatment. However, a decrease in FTS and FTRS was observed, both treatments showed higher values than the ones found in other studies. The values of individual motility, viability, normality, and biological quality were affected by the process of freezing and refreezing, nevertheless, all these exceed the established ranges as seminal samples suitable to be used in artificial insemination. These results suggest that the refreezing technique FTRS is viable in bovine semen.
Keywords:Ejaculate; Endosmosis, Morphology; Motility; Plasma membrane; Viability
Introduction
One of the most recognized techniques for the preservation of the semen of males of different species for an indefinite period of time, is by freezing in liquid nitrogen, a procedure called cryopreservation. However, during the freezing process, thawing can cause irreversible damage to the morphology and functionality of the sperm that could cause cell death. Sperm can undergo a series of biophysical alterations in its nature; being exposed to extreme temperatures of -196 °C when stored in liquid nitrogen, however, the most critical damage does not occur to this temperature, but between -15 °C and -60 °C where changes in the state of freezing and thawing occur, and the sperm must maintain vitality. It is essential that at the same time the optimal rate of temperature drop is considered to avoid cell death Almela [1]. This has been called heat shock of sperm, which results in a decrease in energy and metabolism, loss of intracellular components (due to changes in the concentration of intra and extracellular solutes), increased permeability of the membrane (caused by cell dehydration) and loss of motility Watson [2]. The best quality semen must be used to obtain the expected results. Likewise, the objective is that the semen used maintains its fertilizing ability even after undergoing various treatments such as dilution and freezing Muiño [3]. There are several techniques that help to measure the quality of the semen to be used, among those techniques, some that assess characteristics such as the functional and structural integrity of the plasma and acrosomal membrane are of great importance Santiani [4], since it has been shown that semen samples with high proportions of acrosomal damage have very low fertility in vivo and in vitro Cabrera [5]. The Hypo-Osmotic-Swelling-Test (HOST) analysis is currently the most practical test to define the fertility functions of a sperm. This test exposes the sperm to a hypo-osmotic environment, allowing water to enter the membrane to obtain an osmotic balance. At the same time, the sperm’s tail becomes inflamed and subsequently coiled. This tail curl is the aspect that is observed, and it means that the membrane has a correct integrity, and that the sperm is working correctly Correa [6].
Plasma membrane damage is clearly associated with loss of cell vitality; however, an intact plasma membrane does not always indicate that the cell is viable. The most common procedures used to assess vitality are supravital staining techniques (eosin-nigrosin), which allow the differentiation of live from dead sperm due to the permeability of the plasma membrane to supravital dyes; therefore, those cells that present a functional plasma membrane do not allow the passage of the dye, while if it is altered, the dye penetrates inside the cell and appears stained Bajo [7]. Generally, in animal reproduction, the viability of a sperm can decrease fertilization by up to 50% due to the effect of cryopreservation, subjecting it to a double sperm freezing could compromise the fertilization capacity, and in the event that this is successful, it could lead to low embryonic maintenance Underwood [8]. Although, the purpose of these different tests and analyzes on the semen is to determine whether it will have high fertility or not, and that the semen is capable of guaranteeing this to the producer as long as adequate artificial insemination techniques are used; however, there is no seminal characteristic that alone can predict a bull’s fertility. Likewise, handling and hygiene techniques during collection, storage, transportation and application are key to reproductive success in an artificial insemination program Rosas [9].
Refreezing techniques allow other treatments to be given to semen and achieve greater efficiency in animal reproduction. Sexing techniques of previously frozen semen can be performed that allow the most efficient use of semen, obtaining the offspring of the desired sex Vásquez [10], it can be used as an alternative for sexing semen in genomic resource banks Saragusty [11], in equines research has been carried out with reconfrozen semen with the aim of obtaining a greater number of doses from the same animal to be used in programs such as sperm injection (ICSI) and intra-tubal transfer of gametes McCue [12], in humans refreezing has been carried out in up to three cycles, obtaining good characteristics after them Bandularatne [13]. to be able to repackage semen that has been previously frozen in large volumes. Based on the above, the present investigation was carried out, which had as specific objectives to evaluate the effect of the refreezing processes of bovine semen on the integrity of the plasma membrane, morphology, individual motility and viability of the spermatozoa.
Material and Methods
The research was carried out between November 2018 and September 2019 at the Animal Reproduction Laboratory of the Pan-American Agricultural School, 32km from Tegucigalpa- Honduras C.A., with an average temperature of 24 °C, 800msnm and 1100mm of average annual rainfall. The semen of a 60-month-old Brahman bull, which was collected twice. The electro-ejaculation methodology was applied for the semen collection.
Macroscopic evaluation
Volume measurements (normal range: 5-7mL), color (normal: white, milky white, creamy), odor (typical of the species), pH (normal range: 6.2-6.8) Holý [14]. For this evaluation, the ejaculate was placed in a tempered 15mL polycarbonate tube (38.5 °C).
Microscopic evaluation
Mass Motility (MM): It refers to the ability and vigor to a greater or lesser degree to the movement that the sperm have in a fresh ejaculate. It was performed by placing a drop of fresh undiluted semen on a slide tempered at 38.5 °C and was observed directly at 10X and 20X classifying the movement of the waves according to their vigor. Only ejaculates with a rating of 4 (very good, percentage of MM > 81%) were used for this investigation Madrid [15]. Individual Motility (IM): It is an equally qualitative and subjective evaluation, since the percentage of sperm in a semen sample that show progressive rectilinear movement is calculated; all other movements are considered abnormal. A 10: 100 dilution of semen: 0.9% SSF was prepared, and from this a drop was placed on a slide tempered at 38.5 °C, covered with an equally tempered coverslip. It was observed at 20X and 40X. For the purposes of this research, only semen that presented > 80% of MI classified as Very good was used Zemjanis [16].
Concentration: It reflects the amount of sperm present in a given unit of volume, generally cc or mL. The Minitube® Spermacue spectrophotometer was used.
Biological Quality (BQ): Determines the samples (doses) of frozen semen suitable for use in artificial insemination procedures; It is obtained by multiplying the dose concentration by the percentage of individual progressive post-frozen motility; Doses with values ≥10 × 106 sperm with progressive post-frozen MI/dose are considered approved Rosas [9].
Morphology: A good morphology will largely depend on the fertilization process and on the other hand the ability to tolerate cryopreservation. Every ejaculate will always have, to a greater or lesser degree, a percentage of abnormal sperm typical of the constant processes of spermatogenesis, which ranges between 5-10%. In general, ejaculates with abnormalities lower than 30% are considered an acceptable value Holý [14]. For the head, acrosome, middle and tail stains, the Minitube® Spermac stain was used Minitube [17]. The acrosomes and the equatorial zone are stained light green, while the rest of the head appears in red, and both the tail and the intermediate piece will be dark green. At least 1000 spermatozoa were counted under the phase contrast microscope for each structure/smear and three smears/ejaculate were made.
Viability: It refers to the number of live and dead sperm, so it is directly related to fertility and motility, both mass and individual. For this parameter, the BLOM method (eosin 2% -nigrosin 10%) was applied: On a slide tempered at 38 °C, a drop of semen +2 drops of aqueous 2% eosin solution and a drop of nigrosin 10% were placed, it was left to rest for 10 to 30 seconds, then a drop of the mixture was taken, a smear was made on a degreased slide and tempered to 38 °C, and observed at 40X; The whole process should not take more than 5 minutes, otherwise the live sperm will begin to die; Live sperm will have a white head (not stained) since, being alive, their plasma membrane is intact and this does not allow the entry of the primary dye (eosin) while the dead ones already have damage and/or alterations in the membrane and therefore it allows the entry of the primary dye, staining the head from pink to red. A count of at least 1000 sperm/smear (three smears/ejaculate) was taken; a maximum of 30% deaths is allowed Holý [14].
Endosmosis test: Hypo-Osmotic Swelling Test (HOST), It consists of subjecting the sperm to a medium of lower osmotic pressure than the physiological one, which causes water to enter the interior of the cell (mainly to the tail), leaving it swollen and curled (Positive Endosmosis PE). For this response to occur, the plasma membrane must be intact. Sperm with physical damage or alterations will not undergo changes in the shape of the flagellum (Negative Endosmosis NE). The methodology was: Dilute the semen (100μl) in 900μl of the hypo-osmotic medium (100mOsmol/l), incubate for 60 minutes at 37 °C Bedoya [18], make a smear, fix on plate at room temperature for five minutes and observe under a phase contrast microscope, count at least 1000 sperms per smear (three smears per ejaculate). The higher the PE, the higher the quality of the seminal sample. The preparation of the hypo-osmotic solution (HOST solution) used was the protocol proposed by Campi [19].
Freezing process: For the dilution, the Minitube Triladyl® diluent was used, the concentration at packaging was 30x106 sperm in 0.5cc straws; the process involved dilution, packing, equilibrium at 4 °C for four hours and nitrogen fumes for 10 minutes. Three treatments were applied to each semen collection (each collection was divided into three aliquots): 1) Fresh Diluted Semen (FDS): the semen was diluted in Triladyl®; 2) Frozen-Thawed Semen (FTS): the semen diluted in Triladyl® and frozen at -196 °C, after 48 hours, six straws from each collection were defrosted at 38 °C/45 seconds in a water bath and evaluated; 3) Frozen-Thawed-Refrozen Semen (FTRS): the semen diluted in Triladyl® was frozen at -196 °C, after 48 hours, 20 straws from each collection were defrosted at 38 °C/45 seconds in a water bath, subsequently left at room temperature for 60 minutes, at which they were refrozen, placed at 4 °C (cooling rate -0.5 °C/minute) for 4 hours (equilibrium) and subsequently under nitrogen vapors for 10 minutes (4cm above nitrogen level; average cooling rate -19 °C/minute) and then introduced into liquid nitrogen -196 °C. After 48 hours, ten straws from each batch were thawed at 38 °C/45 seconds and evaluated again. The following variables were measured for the three treatments: individual motility (%), biological quality (#sperm with post-frozen progressive MI / dose), viability (%), morphology (%), sensitivity to the HOST endosmosis test (% of PE and in). A Completely Random Design (CRD) was used with three treatments (FDS, FTS, FTRS) and two repetitions per treatment. The arc-sine test was applied for the conversion of the percentage values and an analysis of variance (ANOVA) using the General Linear Model (GLM) and separation of means with Duncan’s multiple range test; for the correlations, the Pearson Correlation was used using the statistical program “Statistical Analysis Systems” with a required level of significance of p≤0.05.
Result and Discussion
Individual Motility (IM)
The differences were significant (p≤0.05) between the treatments (Table 1), being the FDS treatment the one that obtained the highest percentage, surpassing the FTS and FTRS by 27.08% and 46.25% respectively; These results are attributed to the fact that the FDS did not go through the cryopreservation process. On the other hand, the FTRS presented a 19.17% decrease in IM compared to the FTS. IM was affected, since cryopreservation negatively affects IM, causing damage to the structure of the sperm and cell death Rivera [20]. The FTS exceeded the FTRS by 19.17%, however, despite the fact that the IM was decreased, the two treatments that underwent cryopreservation processes, presented results similar to those reported by Arav [21] of 50% for re-frozen semen and 75% for frozen semen and the minimum of 35% to obtain a classification of the post-thaw biological quality as approved, as long as the concentration is 30 x 106 spermatozoa per dose Rosas [9]. The IM of the FTRS decreased by 46.25%, which is equivalent to 50% of the IM of the FDS and when compared with the FTS it decreased 19.17%. Therefore, with the results obtained in this research, it is concluded that for a semen to be suitable for refreezing and to achieve acceptable semen doses for artificial insemination, it must have been frozen for the first time with an individual fresh motility ≥80% or it must have IM at thaw ≥60%.
Table 1: Percentages of individual motility, biological quality, viability, abnormal acrosomes, and positive endosmosis of fresh bovine semen diluted and subjected to freezing and refreezing.
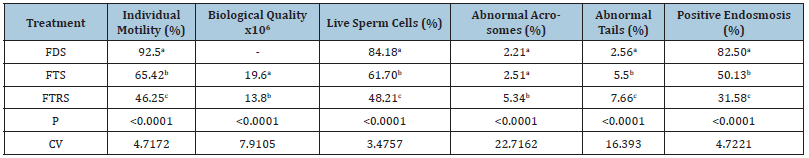
Fresh Diluted Semen (FDS); Frozen-Thawed Semen (FTS); Frozen-Thawed-Refreezing Semen (FTRS). P: Probability; CV: Coefficient of Variation.
Biological quality (BQ)
Significant differences (p≤0.05) were found between FTS and FTRS (Table 1). Variations in biological quality are directly attributed and related to individual motility, however, although the differences were significant, both FTS and FTRS treatments are above the “approved” range established by Rosas [9] of a minimum of 10x106 viable spermatozoa per dose, therefore, the refreezing process can be a viable alternative in the management of bovine semen. For this reason, evaluating the biological quality of semen is of outmost importance since it relates the key aspects of semen fertility: progressive individual motility (involves normal, live sperm) and concentration per dose. This implies that the biological quality of the refrozen semen seems to be influenced by the donor with differences between the bulls, with the BQ of the refrozen semen being higher from bulls that have higher fertility with frozen semen.
Viability
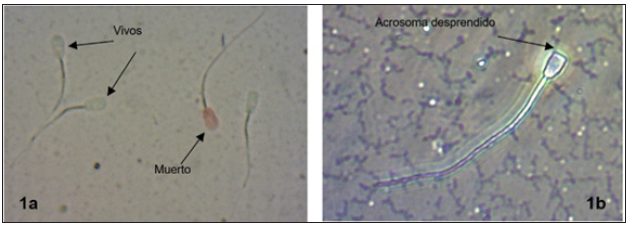
The differences were significant (p≤0.05), being the FDS the one that presented the highest amount of live sperm cells (Table 1); (Figure 1), surpassing the FTS and the FTRS in 22.48% and 35.97% respectively. Similarly, the FTRS decreased by 13.49% of viability when compared to the FTS. The reduction in the number of live sperm is directly related to damage to sperm vitality. Cryopreservation causes severe damage or death of up to approximately 60% of cells; cell death is mainly due to ruptures in the outer plasma membrane that causes a loss of its interaction, forming intracellular ice Bedoya [18]. Under the conditions of this study, freezing and refreezing of bovine semen caused decreases in viability between 22% and 36% compared to fresh semen, respectively, very important values to take into account when evaluating fresh bovine semen for cryopreservation.
Figure 1: a. Sperm viability. The unstained head represents the live sperm, while, in the dead, it is colored pink or deep red. b. Abnormal acrosome
Morphology
For the integrity of the acrosome, the differences were significant (p≤0.05) with values of 2.21%, 2.51% and 5.34% of abnormal acrosomes for FDS, FTS and FTRS respectively, being FDS and FTS similar, but both differ from FTRS (Figure 1), however, all the percentages of sperm with normal acrosomes in all the treatments showed values greater than 90% (Table 1). The main abnormality presented was detached acrosomes. According to Holy [14] alterations at the level of the cranial or equatorial part of the sperm head are normally between 3-5%, but an increase between 10- 30% causes subfertility, the values being found in this investigation within normal parameters. Regarding the morphology of the tails, there were also differences (p≤0.05) with values of 2.56%, 5.50% and 7.66% of abnormal tails for FDS, FTS and FTRS respectively FDS and FTS being similar, but both differ from FTRS. Similarly, all the percentages of sperm with normal tails in all treatments showed values greater than 90% (Table 1). The main abnormalities presented were detached and broken tails. The results in general morphology also presented significant differences (p≤0.05) between the three treatments, however, all treatments presented values higher than 90% of normal sperm. Because the acrosome possesses enzymes that participate directly in the fertilization of the ovum, the artificial insemination industries use the percentage of normal post-thaw acrosomes as a determining factor for fertility Zapien [22]. Similarly, other authors conclude that the alterations of the acrosome and its percentage loss are attributed to the cryopreservation process to which the sample was subjected Rivera [20]. Regarding the tail, it is part of the locomotor system and as such it seeks to obtain as many normal cells as possible since a sperm can only fulfill its function of fertilizing the ovum depending on the correct and normal constitution of the locomotor system (neck, middle and tail) Holy [14]. For this part, Zemjanis [16] recommends that no more than 25% of abnormal tails should be present. However, despite the fact that the FTRS went through two cryopreservation processes, it presents a mean value of 6.54% of abnormalities in general, being well below the maximum values of allowed abnormal sperm of 18% to be considered good quality semen for artificial insemination Lamothe [23].
Positive Endosmosis (PE)
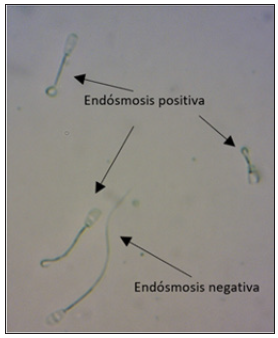
The differences were significant (p≤0.05), being the FDS the one with the highest PE (Table 1); (Figure 2). In the FTRS after undergoing two freezing processes, a 50.92% decrease in the EP was obtained, while the FTS after a freezing process decreased the EP by 32.37%, which shows the harmful effect of the cryopreservation process on the integrity of the sperm plasma membrane and at the same time highlights the importance of selecting high quality semen from the beginning when performing cryopreservation processes. A positive correlation (p≤0.05) was found between IM and EP (r=0.9659); EP and viability (r=0.9764); EP and BQ (r=0.8927); BQ and viability (r=0.8012); BQ and IM (r=0.9999). The FTS treatment presented an adequate PE percentage since studies have shown that a sample must present a PE reaction of at least 40% of the seminal cells to be considered “adequate” Bernardi [24]. However, for the FTRS sample the results were between 31-40%, indicating that refreezing affects the integrity of the plasma membrane. Despite the above, it is important to remember that each bull and ejaculate must be evaluated in all parameters, both macro and microscopic, and these must be correlated with each other, giving at the end an assessment of the seminal quality both fresh and frozen and refrozen. The positive correlations in general show that spermatozoa that have an intact, biochemical and physiologically active plasma membrane, will have a higher and better progressive individual motility, which means a greater probability of fertilization. On the other hand, in this study, 10 encased cows (Holstein x Brahman) were inseminated with the refrozen semen, achieving a pregnancy rate of 40%, results that are similar to other authors Arav [21] who reported pregnancy rates of 45.5% (56/123) and 44% (47/105) with frozen and re-frozen semen respectively, which suggests that the re-freezing technique of bovine semen can be considered as an alternative in biotechnological management processes (sexing of already frozen semen) and cryopreservation of bovine semen.
Figure 2:Note the coiled tail for positive endosmosis and the uncoiled tail for negative endosmosis.
Conclusion
The processes of freezing and refreezing affect the integrity of the plasma membrane and the acrosome, individual motility and viability. Likewise, it was determined that in frozen-thawed and frozen-thawed-refrozen semen the percentages of individual motility and the percentage of live sperm decreases while the percentage of abnormalities increases with respect to fresh semen. The refreezing process of bovine semen should be applied only to seminal samples that have presented individual motility when fresh ≥80%. Likewise, the highest percentage of spermatozoa with positive endosmosis (HOST) was found in fresh semen, however, frozen-thawed semen also presented adequate values, while semen subjected to refreezing had values below those recommended. The biological quality was affected by the freezing and refreezing processes, however, in both treatments the values are acceptable to be used in artificial insemination. Positive endosmosis (HOST) was determined to have a positive correlation with biological quality, individual motility, viability, and morphology. These results suggest that the refreezing technique is viable in bovine semen, therefore it is recommended to carry out more research with different breeds and a greater number of animals [25].
Acknowledgment
The authors thank the Animal Reproduction Biotechnology Laboratory at the University of Zamorano, Honduras for its support both in the logistics and financing of this research.
References
- Almela L (2014) Aportaciones a la criopreservación de gametos masculinos en la raza bovina Murciano Levantina: Recongelación de espermatozoides' Tesis Doctoral, Universidad de Murcia, Spain, p. 192.
- Watson PF (1981) The effects of cold shock on sperm cell membranes. In: Morris GJ, Clarke A (Eds.), Effects of Low Temperatures on Biological Membranes, Academic Press, London.
- Muiño R, Fernández M, Areán H, Viana J, López M, et al. (2005) Nuevas tecnologías aplicadas al procesado y evaluación del semen bovino en centros de inseminación ar ITEA 101(3): 175-191.
- Santiani A, Sandoval R, Ruiz L, Coronado L (2004) Estudio de la integridad de membrana en espermatozoides de ovino mediante la prueba de estrés hipo-osmótico' en Laboratorio de Reproducción Animal, Facultad de Medicina Veterinaria, Universidad Nacional Mayor de San Marcos (eds), XXVII Reunión Asociación Peruana de Producción Animal APPA Piura. Lima, Perú, pp.1-11.
- Cabrera P, Pantoja C (2012) Viabilidad espermática e integridad del acrosoma en semen congelado de toros nacionales. Rev Investig Vet 23(2): 192-200.
- Correa JR, Zavos PM (1994) The hypoosmotic swelling test: Its employment as an assay to evaluate the functional integrity of the frozen-thawed bovine sperm membrane. Theriogenology 42(2): 351-360.
- Bajo Arenas JM, Coroleu Lletget B (2009) Fundamentos de Reproducción. Editorial Médica Panamericana, Madrid, España, Spain.
- Underwood SL, Bathgate R, Maxwell WMC, Evans G (2009) Development of procedures for sex-sorting frozen-thawed bovine spermatozoa. Reprod Dom Anim 44(3): 460-466.
- Rosas J (1997) Determinación de la calidad biológica del semen congelado en Universidad Juárez Autónoma de Tabasco e Instituto Nacional de Investigaciones Forestales y Agropecuarias, Memorias del VI curso de actualización en reproducción animal, Tabasco, México, pp. 25-31.
- Vásquez JM, Parrilla I, Roca J, Gil MA, Cuello C, et al. (2009) Sex-sorting sperm by Flow cytometry in pigs: issues and perspectives. Theriogenology 71(1): 80-88.
- Saragusty J, Gacitua H, Zeran Y, Rozenboim I, Arav A (2009) Double freezing of bovine semen. Anim Reprod Sci 115(1-4): 10-17.
- McCue PM, Moore AI, Bruemmer JE (2004) Refreezing stallion spermatozoa for assisted reproduction. Reprod Fert Develop 16(1): 76-177.
- Bandularatne E, Bongso A (2002) Evaluation of human sperm function after repeated freezing and thawing. Journal of Andrology 23(2): 242-249.
- Holý L (1987) Biología de la reproducción bovina: Introducción al proceso del examen de la fertilidad de la hembra y el macho, Ed. Científico-Técnica, La Habana, Cuba.
- Madrid Bury N (2001) Evaluación de la aptitud reproductiva del toro: Reproducción Bovina, Fundación Girarz, Maracaibo, Venezuela.
- Zemjanis R (1981) Reproducción animal: diagnóstico y técnicas terapéut In: Limusa SA (Ed.), México.
- Minitube (2016) Spermac stain manual, Minitube GmbH, Germany.
- Bedoya NA, Vásquez N, Rivera M, Corre G, Trujillo LE (2002) Evaluación de la integridad funcional de la membrana plasmática de espermatozoides bovinos mediante el test hipoosmótico (HOST). Rev Fac Nal Agr 56(2): 1983-1997.
- Campi S, González L, Blasi C, Suhevic J, Bonet S, et al. (2012) Comparación entre distintos tiempos de lectura en el test de endósmo Laboratorio de calidad seminal y criopreservación. Buenos Aires, Argentina, Universidad de Buenos Aires, Argentina.
- Rivera M (2018) Congelación de semen bovino. Revista Genética Bovina, Consultado,
- Arav A, Zeron Y, Shturman H, Gacitua H (2002) Successful pregnancies in cows following double freezing of a large volume of semen. Reproduction Nutrition Development 42(6): 583-586.
- Zapien A (1997) Capacidad reproductiva y predicción de fertilidad en toros en Universidad Juárez Autónoma de Tabasco e Instituto Nacional de Investigaciones Forestales y Agropecuarias, Memorias del VI curso de actualización en reproducción animal, Tabasco, México, pp. 56-66.
- Lamothe C (1997) Características del eyaculado en Universidad Juárez Autónoma de Tabasco e Instituto Nacional de Investigaciones Forestales y Agropecuarias, Memorias del VI curso de actualización en reproducción animal, Tabasco, México, pp. 12-24.
- Bernardi SF, Allende R, Mazzeo R, Monti J, Marini PR (2011) Evaluación de los cambios ocasionados en espermatozoides bovinos por variaciones en el manejo de las dosis durante su manipulación en inseminación In Vet 13(2): 25-38.
- SAS (2012) User Guide: Statistics. Cary NC, SAS Inst. Inc., USA.
© 2021 Hincapie John Jairo. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






