- Submissions

Full Text
Clinical Research in Animal Science
Production of Parthenogenetic Blastocysts from Oocytes Aspirated of Ovine Ovaries with or without Corpus Luteum
Hincapie JJ1*, Cuellar CJ2, Ross PJ3 and Castillo R4
1Departament of Agricultural Science and Production, Zamorano University, Tegucigalpa, Honduras
2Departament of Agricultural Science and Production, Zamorano University, Tegucigalpa, Honduras,
3Animal Reproduction Department, UC Davis, California, United States
4Departament of Agricultural Science and Production, Zamorano University, Tegucigalpa, Honduras,
*Corresponding author: Hincapie JJ, Departament of Agricultural Science and Production, Zamorano University, Tegucigalpa, Honduras
Submission: February 26, 2021;Published: March 05, 2021

ISSN: 2770-6729Volume 1 - Issue 3
Abstract
The experiment was carried out between January and March 2020 in the animal reproduction laboratory at the University of California, Davis USA. The objective was to evaluate the effect of the presence of the corpus luteum in the aspirated oocytes of ovine female ovaries at different stages of the reproductive cycle, on the parthenogenetic development of embryos in vitro to determine the average number of oocytes collected per ovary, percentage of viability of oocytes, percentages of cleavage and blastocysts in vitro and the efficiency of the procedure. 158 ovaries from sheep from a slaughterhouse were used; mean values of 3.33, 1.24 and 1.52 oocytes / ovary were obtained and the percentages of viability were 100%, 45.87%, and 56.82% for oocytes from ovaries with corpus luteum, ovaries without corpus luteum and ovaries from pregnant females respectively (P≤0.05); there were no differences (P>0.05) in the cleavage percentages with values of 72.5%, 72.0%, and 56.0% and in the parthenogenetic blastocysts with values of 37.93%, 44.44%, and 42.86% for oocytes from ovaries with corpus luteum, ovaries without corpus luteum and ovaries of pregnant females respectively, however, the highest production of blastocysts / ovary (P≤0.05) was from ovaries with corpus luteum. It is concluded that the effect of the presence of the corpus luteum in the aspirated oocytes of ovine female ovaries improves the parthenogenetic development of embryos in vitro when evaluating the production of blastocysts per ovary.
Keywords: Parthenogenic activation; Biotechnology; Embryos; In vitro fertilization
Introduction
Parthenogenesis represents the production of an embryo from a female gamete without
the inclusion of a male gamete. Parthenogenetic activation seeks to restart meiosis since the
oocyte without activation is arrested in the metaphase of the second meiotic division [1].
That means, it seeks to activate all enzymatic processes and restart meiosis and activate the
development process in the absence of fertilization.
The study of parthenogenesis allows us to focus only on the quality of the oocytes, otherwise with in vitro fertilization, the male influences the results. Other authors suggest that oocytes with low developmental capacity have a slower energy metabolism that delays further development. Prepubertal oocytes reached the Metaphase I stage one hour later than adults and this delay increases as the first meiotic division progresses [2].
The culture media supplemented with P4 significantly improved the development of mouse embryos. Furthermore, an in vivo experimental design has demonstrated high blastocyst survival and implantation rates in P4-treated mice [3].
Progesterone seems to affect follicular growth, oocyte maturation, and embryonic development. According to various investigations, progesterone levels and its relationship with estrogen levels are strongly associated with the quality and maturity of oocytes [3]. The degree of effect of part of the hormone will be based on the concentration and the species treated. Based on the above, the present investigation was developed, which had as specific objectives: To determine the average number of oocytes collected per ovary, the percentage of viability of the oocytes and to determine the percentages of cleavage and blastocysts in vitro and as well as evaluate the efficiency of the procedure.
Material and Methods
The research took place between January and July 2020 in the Meyer Hall reproduction laboratory in the Department of Animal Science, at the University of California, Davis in the United States. It is located at 14masl, and presents an annual precipitation of 105mm and a temperature range between 4 °C to 27 °C.
Collection of ovaries
The ovaries were collected from the slaughter plant and transported to the laboratory in 0.9% saline solution with 1% penicillin/streptomycin with a concentration of 10,000IU of penicillin and 10mg/L streptomycin at 30-35 °C. Once in the laboratory, the ovaries were rapidly washed with water at 37 °C to remove excess blood and then placed in a new saline solution in a water bath to stabilize the temperature at 37 °C.
Follicular aspiration
Aspiration was performed using the recovery medium and a vacuum system. The follicular fluid was deposited in 15mL Falcon™ tubes and placed in the thermo block until searching under the microscope. For the recovery medium, the following were used: 20mL of TCM-199+200μl of Penicillin/Streptomycin+ 56.5μl of Heparin (Stock) + 200μl of fetal bovine serum + 0.0119g of HEPES.
Recovery and classification of oocytes
All recovered oocytes were washed three times and were classified into four categories: A, B, C, D. Only grade A and B oocytes were placed in maturation. The criteria used were: Grade A: many compact layers of cumulus oophorus cells, homogeneous cytoplasm; Grade B: partially surrounded by cumulus oophorus cells, homogeneous cytoplasm; Grade C: naked oocytes; Grade D: oocytes surrounded by fibrin.
In vitro maturation
35mm plates were prepared with four 70μl drops of maturation medium covered with 3.5mL of mineral oil, depositing between 25 to 35 oocytes per drop giving a ratio between 2to3μl / oocyte. For the preparation of the maturation medium, the following were used: 4.5mL of TCM-199+50μl of Penicillin/streptomycin + 500μl of sheep serum in heat + 25μl of ovine FSH (50ng/ml stock) + 25μl of ovine LH (3μg/mL) + 5μl of Cysteamine.
Previous preparation of stock solutions
Ovine (NHPP): (0.01mg/mL - 200X) AFP7558C. 0.1mg of FSH was dissolved in 10mL of PBS pre-filtered (pH 7) with BSA-FAF at 1% w/v (A6003). Aliquots of 70μl and stored at -80 °C.
LH (Sioux): (3mg/ml) Sioux 725. 3mg of LH was added to 1ml of PBS prefiltered (pH 7) with 1% w/v BSA-FAF (A6003). Aliquots of 15μl were made and stored at -20 °C.
Cysteamine (10mM; 100X): Sigma M6500. 5.7mg of cysteamine was weighed in 5mL of TCM-199. Everything was prepared on ice. The tubes were pre-cooled. 70μl aliquots were prepared under sterile conditions and stored at -20 °C. The plates were equilibrated for two hours in the incubator before placing the oocytes. In vitro maturation conditions: 24 hours in the incubator, 5% CO2, 38.5 °C and relative humidity saturation.
Parthenogenetic activation
Activation dishes were prepared three hours before use. An aliquot of Di-Methyl-Amino-Purine (DMAP) was placed on the flame until it dissolved. 100mm dishes containing 50μL/ 25 zygotes were prepared using activation or culture medium. These are culture drops. The drops were covered with mineral oil and the plate was placed in the incubator with 5% CO2 and 38.5 °C.
Three drops of oocyte transport medium (SOF-HEPES) were placed, and the oocytes were washed through each drop three times. The zygotes were transferred to 0.6mL of hyaluronidase in a microcentrifuge tube and the volume of hyaluronidase was lowered to 100μl, verifying that COC (cumulus-oocyte complex) had not been aspirated.
Subsequently, the microcentrifuge tube was placed in the vortex for 5 minutes and withdrawn every minute in order to remove the cells from the cumulus oophorus. 1mL of SOF-HEPES was added to the tube, the oocytes were mixed and removed, checking that there were no oocytes in the tube. The number of oocytes to activate was searched and a drop of SOF-HEPES was placed in which they were washed three times.
Then a 1.5mL tube was used, 1μl of ionomycin and 1mL of SOF-HEPES were added and mixed. The oocytes were placed in 1 drop of ionomycin/SOF-HEPES mixture and allowed to stand for four minutes in total darkness. After this time, the oocytes were washed three times in drops of SOF-HEPES and once in BO-IVC + DMAP, they were separated into groups in BO-IVC + DMAP dishes. They were incubated on a plate with KSOM + DMAP medium for 4 hours. After the elapsed time, the oocytes were washed again in 3 drops of SOF-HEPES and once in BO-IVC + BSA.
In vitro embryo culture
The culture medium was prepared three hours before use. 60mm Petri dishes containing 50μl drops of culture medium/25 zygotes were prepared, covered with mineral oil and then the dishes were placed in the incubator with 5% CO2, 38.5 °C and relative humidity saturation. The zygotes were transferred to a culture plate containing 50µl of mineral oil coated culture medium. The dish was placed in a chamber and the gas inside the chamber was replaced with an air mixture of 5% O2, 5% CO2 and 90% N2 for two minutes and then placed back in the incubator at 5% CO2 and 38.5 °C. On day three of the culture, it was supplemented with 2.5μl of Fetal Bovine Serum/drop of culture (Gemini Bio 100-525). On day 7, blastocyst formation was evaluated.
Treatments
Three treatments were developed
1) oocytes from sheep ovaries in the anestrus state (without
corpus luteum)
2) oocytes from sheep ovaries with corpus luteum present.
3) oocytes from ovaries of pregnant ewes.
Variables analyzed
Average of oocytes collected per ovary, percentage of viability of the oocytes according to the number of layers of cumulus oophorus cells and the homogeneity of the cytoplasm, percentage of cleavage at 72 hours, percentage of parthenogenetic blastocysts and general efficiency.
Experimental design and statistical analysis
A Completely Random Design was used with three treatments and two repetitions per treatment. The percentage values were analyzed using the Chi-square (χ2) frequency distribution test; For the numerical values of extracted oocytes and number of oocytes/ovary, an Analysis of Variance (ANOVA) was used applying the General Linear Model (GLM) and Duncan's multiple range test, with a required significance value. of P≤0.05, using the "Statistical Analysis Systems" program [4].
Result and Discussion
Oocytes collected per ovary
The differences were significant (P=0.0001) between the treatments, being the treatment of the aspirated ovaries with corpus luteum the one that obtained the highest number of aspirated oocytes/ovary with a value of 3.33 followed by the ovaries of pregnant females with 1.52 and the smallest for ovaries without corpus luteum with 1.24. These results are attributed to the fact that by presenting the ovary the corpus luteum has as its main action the production of the hormone progesterone, which has an effect on follicular development since it is responsible for inhibiting the dominant follicle, therefore, by generating the Regression of the dominant follicle creates a new wave of follicular development [5]. Sheep have one or two ovulations per cycle and this variation depends on genetics, age, season, and nutrition. In this study, ovaries were taken from a slaughterhouse where the age of the sheep is unknown, but it is known that they were taken to the slaughterhouse during the short days that was the month of January, where they show greater cyclicity and prolificacy.
Percentage of viability
There were significant differences (P≤0.05) between the treatments (Table 1), being the treatment of the aspirated ovaries with corpus luteum the one that obtained the highest number of viable oocytes, surpassing the treatments of ovaries without corpus luteum and ovaries of pregnant females in 54.13% and 43.2% viability respectively. The viability of oocytes is related to the ability of oocytes to generate an embryo, ovaries with corpus luteum have a high percentage of viability. The higher the circulating progesterone, the concentrations promote follicular turnover and with the new wave of ovarian follicular development, it results in a viable oocyte that is released [6]. According to studies carried out by Menchaca et al. [6] mention that by affecting the competition of the oocytes with progesterone, they generated viable embryos. What is attributed to the fact that the results of the present investigation when comparing the treatments, ovaries with corpus luteum have given greater results than the other treatments.
In the present investigation, oocytes with many compact layers of cumulus oophorus cells and homogeneous cytoplasm were taken, which is an indicator of high quality oocytes, that is, they will be viable for the production of embryos in vitro and according to a study carried out by Córdova et al. [7] follicles larger than 3mm have more layers of cumulus, which generates better maturation in vitro, being an important aspect to take into account when selecting oocytes at the end of their growth phase. This is attributed to the fact that the oocytes will have nutrients and energy to be able to develop better compared to others that are grade C and D, since these oocytes are discarded because they do not have enough layers of cumulus oophorus and are not suitable for use in embryo production.
According to Lonergan [8], the quality of the oocyte is based on different aspects such as the physiological and reproductive state of the donor, the follicular size and the amplitude and integrity of the cumulus cells. In this study the breadth and integrity of the cumulus cells is known, and these played an important role for the maturation of the oocytes since they provide energy and nutrients to the oocyte, in this study oocytes with high quality cumulus layers were selected along it is attributed that the results have provided a percentage greater than 50%.
The morphology of the aspirated oocytes from the ovaries allows the possibility of predicting their ability to restart meiosis [9]. When analyzing the three treatments, it is observed that the oocytes from the ovaries with corpus luteum stood out since at the time of selecting and classifying them all were viable, these results are attributed to the cyclical activity that these sheep presented in vivo, since they were in the riding season which is characterized by short days resulting in greater cyclicality.
Cleavage percentage
The differences found were not significant (P>0.05) between the treatments (Table 1), with all treatments exceeding 50% cleavage. These results are good because in all treatments they presented cleavage greater than 50%, which may mean better results for the formation of a viable blastocyst.
The cleavage of oocytes is important since it means that when the cells are dividing, the creation of blastocyst can be achieved [5]. This study sought to analyze the production of blastocysts by parthenogenetic activation that are linked to the cleavage of oocytes. When analyzing the results, it was determined that the oocytes independent of the treatment presented a cleavage of more than 50%, but an effect was not obtained that differentiates whether a treatment is significant compared to the others, so it is inferred that the selection and classification of the oocytes play an important role in the competition of oocytes and thus in obtaining acceptable cleavage rates. Although the treatment of oocytes from ovaries with corpus luteum showed the highest viability, this effect was neither reflected nor maintained in cleavage.
Percentage of parthenogenetic blastocysts
There were no significant differences (P>0.05) between the treatments (Table 1), in general, all treatments exceeding 35% of parthenogenetic blastocyst production. According to studies carried out by Menchaca et al. [6] blastocyst production trends have been established, with 50% of the oocytes generating blastocysts. When comparing with the present study, lower results (27.85%) were obtained than those obtained by this author.
Table 1:Mean values of viable oocytes, cleavage, and blastocyst rate of sheep, according to the physiological condition of ovaries with corpus luteum, without corpus luteum and pregnant.
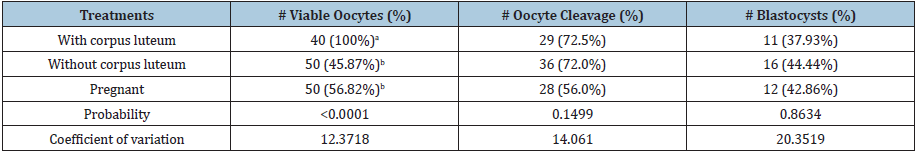
abAverage values in columns with different letters, differ statistically from each other (P≤0.05).
In vivo cultured, fertilized oocytes yield between 60 and 70% blastocysts, but when in vitro matured blastocysts are used, the development rate is 50%, according to a study by Córdova et al. [7]. If the objective is to generate the greatest number of blastocysts, it is necessary to select competent oocytes for in vitro maturation, fertilization and development, fulfilling this condition only grade A and B oocytes, however, this varies greatly depending on genetics and ambient.
The embryos produced by parthenogenetic activation are used in order to generate high-quality information to improve in vitro production techniques in small ruminants such as sheep, since it should be considered that only the reproductive capacity of female sheep is evaluated. and at the time of making the selection the oocytes must be taken primary care since this will affect the whole process.
According to studies by Zhu et al. [10] showed that advances in in vitro production in sheep by new techniques have improved the entire process to obtain quality oocytes. As there were no studies carried out in sheep, it was decided to compare with the study carried out by Lonergan [11] who, when evaluating P4 in cattle, mentions that this hormone can indirectly affect oocyte quality through its effects on pulsatility of LH and the development of a persistent dominant follicle. When comparing these results with the present investigation there are no significant differences with the corpus luteum that generates progesterone to affect the production of blastocysts.
General efficiency
The differences found were not significant (P>0.05) for the relationships between the number of parthenogenetic blastocysts produced according to the viable oocytes collected, the number of parthenogenetic blastocysts produced with the cleaved oocytes, however, for the relationship parthenogenetic blastocysts produced by There were significant differences (P≤0.05), with the treatment with the corpus luteum being the one that obtained the highest values, surpassing the treatments without corpus luteum and pregnant women in 73.48% and 70.98% respectively (Table 2), which shows that the stage physiological condition in which the ovary is at the moment of being aspirated, if it influences the number of oocytes aspirated, the viability and the number of parthenogenetic blastocysts obtained.
Table 2:Mean values for the general efficiency of the procedure in relation to blastocysts: viable oocytes; blastocysts: cleavage oocytes and blastocysts: ovary in oocytes from sheep ovaries according to the physiological condition of ovaries with corpus luteum, without corpus luteum and pregnant.

abAverage values in columns with different letters, differ statistically from each other (P≤0.05).
Therefore, based on the results obtained, the most advisable thing would be to aspirate only ovaries that present corpus luteum, however, the difficulty would lie in being able to exclusively acquire this condition, since in slaughter plants the sheep enter indistinctly at any stage of the estrous cycle, however, another alternative would be to perform heat synchronization processes prior to sacrifice or laparoscopically guided follicular aspiration, thus achieving that a group of sheep can have the corpus luteum as the dominant structure in their ovaries at the time of aspiration.
The quality of the oocyte is one of the factors that most affect the performance and production of blastocysts [12]. The quality of the oocyte is influenced by many factors (race, age, prolificacy of the females), however, one of the main ones is the correct classification of the oocytes, since by using only those of class A and B, and discarding all those of class C and D, a better development and competence of the oocytes in the process of maturation and in vitro fertilization can be expected, which would mean a higher percentage of blastocysts.
Conclusion
The ovaries with the presence of the corpus luteum presented higher production of parthenogenetic blastocysts, that is, that the physiological stage in which the ovary is at the moment of being aspirated does influence the amount of oocytes aspirated, the viability and the number of parthenogenetic blastocysts obtained.
Acknowledgment
The authors thank the Meyer Hall Reproduction Laboratory in the Department of Animal Science, at the University of California, Davis in the United States and the Animal Reproduction Biotechnology Laboratory at the University of Zamorano, Honduras for their support both in the logistics part as funding for this research.
References
- Vallejo J, Gómez V, Tarín JJ (2003) Inducción de la partenogénesis en ovocitos de mamí Revista Iberoamericana de la Fertilidad 20(3): 177-187.
- Leoni G, Palmerini G, Satta V, Succu S, Pasciu V, et al. (2015) Differences in the kinetic of the first meiotic division and in active mitochondrial distribution between prepubertal and adult oocytes mirror differences in their developmental competence in a sheep model. PLoS ONE 10(4): e0124911.
- Salehnia M, Zavareh S (2013) The effects of progesterone on oocyte maturation and embryo development. Int J Fertil Steril 7(2): 74-81.
- SAS® (Statistical Analysis Institute Inc) (2013) Statistical Analysis System 9.4 for Windows Standard version users Guide.
- Hafez B, Hafez E (2013) Reproduction in farm animals. (7th edn), Lalit Printer & Binder, New Delhi, India, p. 519.
- Menchaca A, Cuadro F, Dos Santos Neto PC, Bosolasco D, Barrera N, et al. (2018) Oocyte developmental competence it is improved by relatively greater circulating progesterone concentrations during preovulatory follicular growth. Animal Reproduction Science 195(1): 321-328.
- Córdova A, Córdova M, Córdova C, Guerra J (2008) Procedimientos para aumentar el potencial reproductivo en ovejas y cabras. Revista Veterinaria 19(1): 67-79.
- Lonergan P, Monaghan P, Rizos D, Boland MP, Gordon I (1994) Effect of follicle size on bovine oocyte quality and developmental competence following maturation, fertilization, and culture in vitro. Molecular Reproduction Development 37(1): 48-53.
- Chávez J (2017) Efecto del suero de oveja súper ovulada sobre la maduración y fertilización in vitro de ovocitos de ovino. Universidad del Altiplano, Mexico.
- Zhu J, Moawad AR, Wang CY, Li HF, Ren JY, et al. (2018) Advances in in vitro production of sheep embryos. International Journal of Veterinary Science and Medicine 6(Suppl1): S15-S26.
- Lonergan P (2011) Influence of progesterone on oocyte quality and embryo development in cows. Theriogenology 76(9): 1594-1601.
- Rizos D, Ward F, Duffy P, Boland MP, Lonergan P (2002) Consequences of bovine oocyte maturation, fertilization, or early embryo development in vitro versus in vivo: Implications for blastocyst yield and blastocyst quality. Molecular Reproduction Development 61(2): 234-248.
© 2021 Hincapie JJ. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






