- Submissions

Full Text
Clinical Research in Animal Science
The Effect of Intake of Rhizophora racemosa (Red Mangrove) Leaves and Root-Bark on Some Blood Parameters of Broilers
Shidi SA*, Wekhe SN, Amakiri AO and Owen OJ
Department of Animal Science, Rivers State University, Port Harcourt, South-South, Nigeria
*Corresponding author: SA Shidi, Department of Animal Science, Rivers State University, Port Harcourt, South-South, Nigeria
Submission: January 20, 2020;Published: February 12, 2020

ISSN: 2770-6729Volume 1 - Issue 1
Abstract
The effect of Rihizophora racemosa (Red mangrove) on some blood parameters was evaluated in this experiment using the leaf and rootbark. Using a 2x3 factorial arrangement, a completely randomized design experiment was conducted. One hundred and five (105) day old Ross broiler chicks were randomly distributed in seven treatments: A=control, B1=70g leaf, B2=80g leaf, B3 90g leaf C1=70g root-bark, C2=80g root- bark C3=90g root-bark/kg of feed. The experiment lasted eleven weeks. The results wed that additive level of R. racemosa leaf and root-bark significantly (p<0.05) improved blood parameters (PCV, RBC, WBC, Hb) of broiler. It was concluded that R. re can be used as a feed additive in the feed of broilers to improve resistance to abeam ad disease and decreased morbidity and mortality.
Keywords: Blood; Broilers; Leaf; Mangrove; Rhizophora racemose; Root-bark
Introduction
The percentage of animal protein per day per average Nigerian is relatively lower in comparison to his counterpart in developed countries [1]. The problem of food availability on sustained basis has been the major concern of livestock industries in Nigeria. It has been made more critical by the prevailing high competition existing between man and animals for plant products which form food sources for both [2]. This problem has led to reduced livestock numbers and consequently a drop minima) protein availability from beef, pork, mutton and poultry meat. In bridging the animal protein gap in Nigeria, the broiler is considered a feasible option. This is because in the livestock and meat industry, broiler production has the highest and the quickest rate of turn over, and therefore funds invested in the sub-sector could be recouped faster than other livestock enterprises [3].
The increasing animal protein demand problem has geared researchers towards the production of animals that attain maturity earlier and cheaper than hitherto. Attention is drawn to the improvement of broilers production by administering additives. These additives could be synthetic, biological, or of mineral sources, but primarily to improve the growth and other performances of the livestock [4]. Ogebe [5], holds that the solution to the problem of feed or medical resources development must be sought through the careful identification and testing of the numerous indigenous plants species which abound in the tropics. Rhizophora racemosa (Red mangrove) is the red species of the mangrove plants. It is the vast and abundant red mangrove plant among the common trees of the salt-water swamp. This tree develops many stilt roots from the main stem and branches. It is very abundant in the Niger- Delta Region of Nigeria and is highly underutilized. Wekhe [6] using 10, 20 and 30g/kg feed reported that mangrove plants are rich sources of vitamin and mineral, and recommended further research using higher doses. Tanning are economically important agents for synthesis of some medicines and are valuable sources of dyes. The effects of R. racemosa leaves and root-bark on PCV, RBC, WBC and Hb are therefore investigated using 70, 80, and 90g/kg feed.
Material and Methods
One hundred and five (105) Ross day old broiler chicks (DOC) were used. The study was completely randomized using 2x3 factorial design. Two treatment combinations, viz: the R. racemosa leave and root-bark were ad, and three different graded levels were administered (70, 80 and 90g/kg of feed) for both leaf and root-bark, while the control was 0.00g/kg. The arrangement is therefore: Treatments B1, B2 and B3 which had 70, 80 and 90g of dried powdered leaves in 1kg feed, and treatments C1, C2 and C3 had 70,80 and 90g of the dried powdered root-bark in 1kg feed. Treatment A is the control, so had no additive in the feed. The R. racemosa specimens of leaves and roots were obtained from the brackish water of the Eagle Island Port Harcourt. They were flushed clean with tap water allowed air dry of water, they were then separately (i.e. leaf and root) dried in the oven at a temperature of 70/800C for 48hrs to a water content level of 10%. The dried specimens were then ground into powdery forms. These powders were measured in graded doses and fed to the birds as already, described.
The birds were brooded and allowed to acclimatize and stabilize for two weeks. The experiment proper started in their third week of age and lasted for nine weeks. The birds were eleven weeks old at the end of the experiment, when they were slaughtered. Twelve hours prior to slaughter, feed was withheld from the birds to reduce gut content but were allowed tee access to clean drinking water. The birds were killed by decapitation using a sharp knife. Rapid and complete bleeding was ensured by holding the legs of the birds upwards and the head downward. Blood samples of the slaughtered birds were collected into EDTA (labeled) for blood analysis. The tubes were titled gradually and repeatedly to ensure proper mixing and thereby preventing clothing. The Wood samples were immediately taken to the Hematology Department of University of Port Harcourt Teaching Hospital (UPTH) for analysis. The sales were analyzed for red blood cells count, packed cell volume, hemoglobin and white blood cells count. Result obtained from the blood analysis were computed statistically using ANOVA and Duncan’s Multiple Range Test for Separation of means.
Result and Discussion
The Rbc increased significantly almost twice the level of the control (Table 1) in both leaf and root-bark at the highest dosage of 90g/kg feed. This would also mean doubling the potency of the function of Rbc. Both the Pcv and the hemoglobin are also measures of the quantity and quality of the Rbc. So both of them are all inclusive in the functions of rbc which are: citation of gases or respiration; regulation of acid-base balance; nutrient hormone and enzyme transport; transport of cellular wastes to kidneys, liver, lungs and sweat glands for removal from the body; stoppage of bleeding; regulation of body temperature and regulation of body fluids. The superfluous availability of red blood cells means a state of well-being for the body increased energy production for work by the increased oxygen carrying capacity of hemoglobin with consequently productivity due to iced metabolism of nutrients.
Table 1: Effect of R. racemosa leaves and root-bark on blood parameters (g/kg).
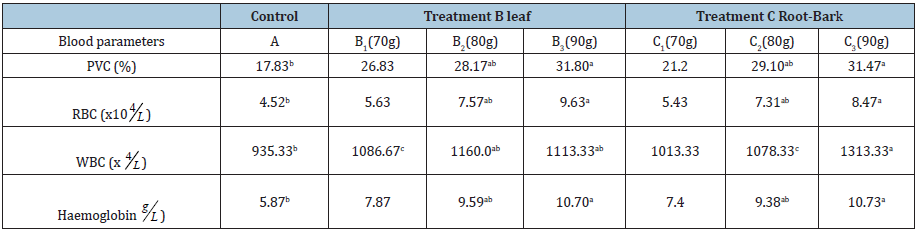
abcmeans along the same row with different superscript are statiscally different (p>0.05).
Packed cell volume (PCV) is the fraction of whole blood volume that consist blood cells (RBC). After centrifugation, the height of the red cell column is measured and compared to the total height of the column of whole blood. The percentage of the total blood volume occupied by the red cell mass is the packed cell volume (PCV). In animals, the protein makes up about 90-95% of the red blood cell’s (RBC) dry content, and around 35% of the fresh content which includes water [7]. RBC are responsible for transporting oxygenated blood and removal of carbon dioxide from the body of the animal. Hemoglobin is the iron-containing oxygen transport metalloprotein in the red blood cells of the birds. It has an oxygen binding capacity of between 1.36 and 1.37ml 02 per gram of hemoglobin [8], which increases total blood oxygen capacity seventy-fold. White blood cells (WBC) are cells of the immune system defending the body against both infectious diseases and foreign materials. Such an increase means enhanced survivability and livability resulting to significantly reduced morbidity and mortality.
All the blood parameters measured increased significantly (P>0.05) when compared with the control. These findings except for white blood cell is in agreement with that reported by Wekhe [9]. The study found no adverse effect of R. racemosa dietary additive on these blood parameters (PCV, RBC, WBC and Hb). The increase in PCV and RBC implies greater supply of oxygen and nutrients to broilers body tissues which consequently manifested in the final body weights. In the other hand, the supply and distribution of oxygen and nutrients would provide energy and support good health. This will in turn promote survivability and livability of broilers [10-13]. The significant differences from the control were more prominent, definite and uniform in the highest dose of 90g/ kg feed. This acceptable as the effective dose and all discussions care on 90g/kg feed. Therefore, prescriptions for improved performance, anti-stress resistance against potential infectious, survivability, livability, minimal morbidity and morbidity of flock should be based on 90g/kg feed [14-16].
Conclusion
It is deducible from the results of the research that R. racemosa can be used as a feed additive to improve performance, reduce morbidity and mortality in a flock. It is suggested therefore that R. racemosa leaf and root-bark be extensively researched in order to reap its plenty benefits.
References
- FAO (1996) Food and Agricultural Organization. The State of Food and Agric, Rome, Italy, pp. 205-2 13.
- Central Bank of Nigeria (1998) Agricultural credit guaranteed fund.
- Smith AJ (1990) Poultry the tropical Agriculturist. CTA, Macmillan, New York, USA, pp. 39-43.
- Randel IK (1979) Growth promoter, the High Mileage Ingredients. Poultry World Magazine, p. 16.
- Ogebe PO (1987) The Influence of lucaema Leucocephala supplementation on the initialization of cassava peels by sheep and goats. AM Sc thesis Department of Animal Science, University of Ibadan, Nigeria, pp. 3-10.
- Wekhe SN, Ebiye A (2007) The effect of Rhizophora racemosa dietary additive on some blood parameters of broilers. 12th Annual Conference of Animal Science Association of Nigeria. Ife, Nigeria, p. 46.
- Portsmonth J (1978) Practical poultry keeping. (7th edn), Spur 7 Publication, London, UK.
- Frandson RD (1975) Anatomy and physiology of farm animals. (2nd edn), Lea and Febiger, Philadelphia, Pennsylvania, USA, pp. 136-146.
- Wekhe SN, Oboh CC (2007) The effect of Rhizophora racemosa (mangrove) feed additive on broiler performance. 3rd Annual Conference of the Nigerian Society for Animal Production. Calabar, Nigeria, pp. 419-521.
- Gous RM, Cherry P (2004) Effects of body weight at, and lighting regimen and growth curve to, 20 weeks on laying performance in broiler breeders. Br Poult Sci 45(4): 445-452.
- Joseph NS, Robinson FE, Renema RA, Thorsteinson KA (2003) Comb growth during sexual maturation in female broiler breeders. J Appi Poult Sci 12(1): 7-13.
- Lewis PD, Backhouse D, Gous RM (2004) Constant photoperiods and sexual maturity in broiler breeder pullets. Br Poult Sci 45(4): 557-560.
- McGary S, Estevez I, Bakst MR (2003) Potential relationship between physical traits and male broiler breeder fertility. Poult Sci 82(2): 328-337.
- Parker TH, Ligon JD (2002) Dominant male red judgiefowl (Gallus gallus) test the dominance status of other males. Behav Ecol Sociobiol 53(1): 20-24.
- Pizzari T, Cornwaffis CK, Lovlie H, Jakobsson S, Birkhead TR (2003) Sophisticated sperm allocation in male fowl. Nature 426(6962): 70-74.
- Zeller FJ (1971) The effects of testosterone on dihydrostestosterone on the comb, and pituitary gland of the female fowl. J Reprod Fert 25(1): 125-127.
© 2020 SA Shidi. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






