- Submissions

Full Text
Clinical Research in Animal Science
Equine Sperm Selection
Gustavo R Larentis* and Henrique BA Bastos
Faculdade de Veterinária, Brasil
*Corresponding author:Gustavo R Larentis, REPROLAB, Faculdade de Veterinária, Porto Alegre, RS, 91540-000, Brasil
Submission: November 21, 2018;Published: June 14, 2019

ISSN: 2770-6729Volume 1 - Issue 1
Abstract
Stallions become sires primarily based on pedigree, athletic prowess and phenotype, with little consideration of reproductive soundness. Inseminating only high-quality spermatozoa selected prior to insemination should improve pregnancy rates. The sperm selection goal is to have a measurable improvement of quality and quantity of spermatozoa when compared to the original ejaculate, while making sure that the process does not impact the integrity of the sample. While several sperm selection techniques are available, the only technique regularly used in commercial equine reproduction programs is the colloid centrifugation technique. Migration methods utilize sperm motility as the main selection factor, where the swim-up method is the most used in humans. Glass wool and Sephadex gel are examples of filter techniques related to frozen-thawed stallion semen. Recently an approach combining the use of a porous membrane and the migration capability was suggested for fresh semen. To this date, none of the techniques described were able to accomplish the goals of an ideal sperm selection, suggesting that further investigation, development and research of new procedures must continue in order to have the best methods to select the fittest spermatozoa.
Keywords: Centrifugation; Porous membrane; Artificial insemination; Fertility; Filters
Introduction
Stallions become sires primarily based on pedigree, athletic prowess and phenotype, with little consideration of reproductive soundness. The equine reproductive industry tends to rely on sires whose fertility is at best, a secondary characteristic [1,2]. Although the minimum number of spermatozoa in an insemination dose is likely to suffer individual variation [3], the reference for mare fertilization was established at 500million sperm with progressive motility (PMS). In a typical artificial insemination (AI) program this dose ranges from 250- 500 million PMS, but recent studies have indicated that doses below 100million PMS do not reduce fertility [4,5]. Several methods have been suggested to mimic the selection of superior spermatozoa in the mare reproductive tract: the filtration of the spermatozoa (active or passive), from the seminal plasma; mimicking the migration of spermatozoa to the uterine horns; or the in vivo process that occurs in the uterus-tubal junction that selects the fittest sperm from the remnant of the ejaculate [6]. In assisted human reproduction the colloid centrifugation and swim-up are laboratory techniques routinely used for the exclusion of immobile and abnormal ejaculate sperm. This processing is performed to obtain high quality inseminating doses [7]. A device using filtration and migration techniques combined was developed recently [8], dispensing deleterious factors involved in centrifugation. The sperm selection goal is to have a measurable improvement of quality and quantity of spermatozoa when compared to the original ejaculate, while making sure that the process does not impact the integrity of the sample [9]. Inseminating only high-quality spermatozoa selected prior to insemination should improve pregnancy rates [6,10]. The objective of this paper is to review what has been published on this interesting subject called sperm selection in stallions, raising some questions and answering others about the techniques described below
Sperm Selection Methods
Colloid centrifugation
While several sperm selection techniques are available, the only technique regularly used in commercial equine reproduction programs is the colloid centrifugation technique [11]. This technique centrifuges extended semen through layers of a varied-density colloid, which promotes separation of the plasma and the viable sperm cells [6]. During this process, the cells will move to the isopycnic point, or the equilibrium position. At this point, the cells can be separated from the plasma [12], not being a physiological method of separation [13]. A disadvantage of this technique is that sperm cells are fragile when you look at the effect’s centrifugation can have on the integrity of spermatozoa, as described in previous publications [14,15]. Another problem detected is the number of samples that must be prepared for commercial use [6]. Two examples of discontinuous density gradient are: RediGrad [16] and EquiPure [17]. An alternative on this method is the single layer centrifugation, which obtained results like those obtained in centrifugation of different layers. This method allows the processing of a larger volume of the ejaculate at a time [18]. A commercial example of this technique is Androcoll-E [19].
Migration
The migration method utilizes sperm motility as the main selection factor, where the swim-up method is the most used in humans [20]. This technique consists of centrifuging semen until it generates a cell pellet, and the motile sperm migrate from this pellet into supernatant medium, from which they are then collected [20]. The motility of the ejaculate samples is greater when using the swim-up technique than when using the centrifuge gradient technique [13]. The main disadvantage of migration methods is the low sperm retrieval [21], making it unfeasible to use this technique in the preparation of inseminating doses in most animal species [6].
Filters
Different techniques of selection of viable spermatozoa were developed in order to retain low quality sperm cells. Glass wool filter, Sephadex gel, and porous membranes are examples of filtering techniques in different species [22]. In pony stallions, a significant increase of seminal parameters is shown when using the glass wool filter [23]. This filter bonds the corpuscular content (proteins, immobile spermatozoa, and rounded cells of other origins) to surface fibers of the glass wool filter. If the fiber mesh is too dense, the cells will still adhere to the surface, but will not properly permeate the glass wool fibers [9]. The combination of glass-wool and Sephadex filtering for frozen stallion semen has proven to have a high correlation with fertility, because stallion membrane proteins give capacitated stallion sperm the ability to bind to Sephadex [24,25]. The higher correlation of glass-wool and Sephadex with fertility is due to the ability of the filters to remove cells that show capacitation-like changes or membrane impairment from the freeze-thaw process [26]. Porous membrane filters were not exactly a sperm selection technique. They were developed to avoid the centrifugation process and increase sperm concentration through the passage of the seminal fluid, retaining the spermatozoa [27,28].
Migration/filtration
Recently an approach combining the use of a porous membrane and the migration capability was suggested for fresh semen [8]. This technique consists of a device divided in half by a porous membrane, and the motile spermatozoa actively cross this membrane (Figure 1). Because the selection method needs no centrifuge or laboratory equipment, it has proven to be an effective and feasible option for sperm selection [8]. Spermatic membrane integrity and functionality, as well as enhanced kinetics were observed after using the selection device [8]. AI is the basis for other biotechnologies ranging from embryo transfer to cloning [29]. To achieve successful pregnancy using AI, enough viable spermatozoa capable of reaching the fertilization site are required [18]. Selecting the healthiest spermatozoa from a raw semen sample improves the quality of the AI dose, and is therefore more likely to achieve fertilization [6]. The ideal sperm separation technique was postulated to
a. be quick, easy and cost-effective,
b. isolate as many motile spermatozoa as possible,
c. not cause damage to the sperm, or non-physiological changes to the separated sperm cells,
d. eliminate dead spermatozoa and other cells, including leukocytes,
e. eliminate toxic or bioactive substances like decapacitation factors or reactive oxygen species (ROS), and
f. allow processing large volumes of ejaculates [30].
Figure 1:Schematic drawing of filtration/migration device.
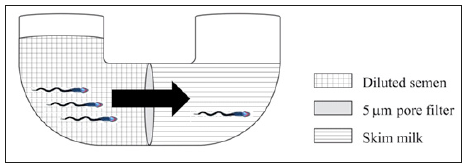
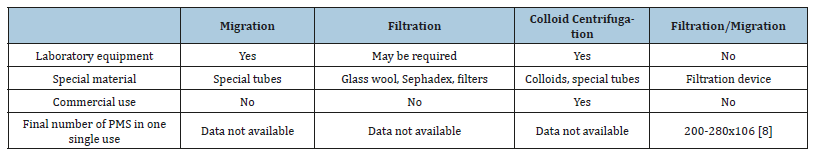
To this date, none of these techniques described were able to accomplish these goals (Table 1). In the selection procedures mentioned above, one or more centrifugation stages are required to enrich the spermatozoa in a desired sample [9]. A major problem of centrifugation is the loss of approximately 25% of spermatozoa in the supernatant liquid [31]. Adverse effects in stallion semen after centrifugation have also been described [14,15]. Damaged and abnormal spermatozoa produce larger quantities of ROS which can contribute to reduced fertility, or problems associated with semen preservation [32]. Sperm membranes and DNA damage can be caused by oxidative stress, eventually converting the sperm into a non-viable spermatozoon. Damaged sperm has been shown to alter embryo development in assisted reproductive technology in humans [33]. Filters have been developed for the removal of seminal plasma, and the concentration of spermatozoa, in an attempt to avoid the issue of plasma membrane damage that centrifugation causes [28].
Table 1:Comparison of the main techniques of the four principles described.
Conclusion
Further investigation and development of the methods presented in this review, and the idealization of new procedures must continue in order to have a better method of selecting the fittest spermatozoa.
Acknowledgement
The authors are grateful to L Onoa for the English revision.
References
- Colenbrander B, Gadella BM, Stout TA (2003) The predictive value of semen analysis in the evaluation of stallion fertility. Reprod Domest Anim 38(4): 305-311.
- Varner DD, Gibb Z, Aitken RJ (2015) Stallion fertility: a focus on the spermatozoon. Equine Vet J 47(1): 16-24.
- Sieme H, Bonk A, Hamann H, Klug E, Katila T (2004) Effects of different artificial insemination techniques and sperm doses on fertility of normal mares and mares with abnormal reproductive history. Theriogenology 62(5): 915-928.
- Brisko SP (2006) Insemination doses: how low can we go? Theriogenology 66(3): 543-550.
- Brisko SP (2011) Semen collection techniques and insemination procedures. In: Mckinnon AO et al. Equine Reproduction, (2nd edn), Chichester: Wiley-Blackwell, Hoboken, New Jersey, USA, pp. 1268-1277.
- Morrell JM, Martinez RH (2009) Biomimetic techniques for improving sperm quality in animal breeding: a review. The Open Andrology Journal 1: 1-9.
- Amann RP, Hammerstedt RH (2002) Detection of differences in fertility. Journal of Andrology 23(3): 317-325.
- Larentis GR, Camozzato GC, Bastos HBA, Gregory RM, RC Mattos et al. (2018) Equine sperm selection by synthetic membrane filter. Journal of Equine Veterinary Science 63: 69-73.
- Engel S, Weber H, Petzoldt R, Seidl B, Wiehe W et al. (2001) An improved method of sperm selection by glass wool filtration. Andrologia 33(4): 223-230.
- Nie GJ, Johnson KE, Wenzel JG (2003) Pregnancy outcome in mares following insemination deep in the uterine horn with low numbers of sperm selected by glass wool/Sephadex filtration, Percoll separation or absolute number. Anim Reprod Sci 79(1-2): 103-109.
- Morrell JM (2012) Stallion sperm selection: past, present, and future trends. Journal of Equine Veterinary Science 32(8): 436-440.
- Pertoft H (2000) Fractionation of cells and subcellular particles with Percoll. J Biochem Biophys Methods 44(1-2): 1-30.
- Mehmood A, Anwar M, Naqvi SM (2009) Motility, acrosome integrity, membrane integrity and oocyte cleavage rate of sperm separated by swim-up or Percoll gradient method from frozen-thawed buffalo semen. Anim Reprod Sci 111(2-4): 141-148.
- Sieme H, Martinsson G, Rauterberg H, Walter K, Aurich C et al. (2003) Application of techniques for sperm selection in fresh and frozen-thawed stallion semen. Reprod Domest Anim 38(2): 134-140.
- Aurich C (2008) Recent advances in cooled-semen technology. Anim Reprod Sci 107(3-4): 268-275.
- Mari G, Castagnetti C, Rizzato G, Mislei B, Iacono E (2011) Density gradient centrifugation of sperm from a sub fertile stallion and effect of seminal plasma addition on fertility. Anim Reprod Sci 126(1-2): 96-100.
- Morató R, Soares JM, Orero G, Mogas T, Miró J et al. (2013) Pre-selection by double layer density gradient centrifugation improves the fertilizing capacity of frozen-thawed, capacitated stallion sperm. Anim Reprod Sci 139(1-4): 62-68.
- Morrell JM, Martinez HR, Johannisson A (2010) Single layer centrifugation of stallion spermatozoa consistently selects the most robust spermatozoa from the rest of the ejaculate in a large sample size. Equine Veterinary Journal 42(7): 579-585.
- Morrell JM, Richter J, Martinsson G, Stuhtmann G, Hoogewijs M et al. (2014) Pregnancy rates after artificial insemination with cooled stallion spermatozoa either with or without single layer centrifugation. Theriogenology 82(8): 1102-1105.
- Metha A, Sigman M (2014) Identification and preparation of sperm for ART. Urologic Clinics of North America 41(1): 169-180.
- Casey PJ, Robertson KR, Liu IK, Espinoza SB, Drobnis EZ et al. (1993) Column separation of motile sperm from stallion semen. J Androl 14(2): 142-148.
- Mogas T, Rigau T, Piedrafita J, Bonet S, Rodríguez Gil JE (1998) Effect of column filtration upon the quality parameters of fresh dog semen. Theriogenology 50(8): 1171-1189.
- Pessoa GA, Martini AP, Trentin JM, Minela T, Fiorenza MF et al. (2017) Response to cooling of pony stallion semen selected by glass wool filtration. Andrologia 49(10).
- Samper JC, Crabo BG (1993) Assay of capacitated, freeze-damaged and extended stallion spermatozoa by filtration. Theriogenology 39(6): 1209-1220.
- Samper JC, Hamilton DW, Pryor JL, Loseth KJ (1995) Mechanism of Sephadex trapping of capacitated stallion spermatozoa. Biology of Reproduction 4(1): 729-737.
- Neild DN, Gadella BM, Agüero A, Stout TA, Colenbrander B et al. (2005) Capacitation, acrosome function and chromatin structure in stallion sperm. Anim Reprod Sci 89(1-4): 47-56.
- Alvarenga MA, Marini MC, Carlos Ona ML, Ozanam PF (2010) A new method to concentrate equine sperm. Animal Reproduction Science 121(1-2): 186-187.
- Alvarenga MA, Papa FO, Carmo MT, Kievitsbosch T, Castro Chaves MMB et al. (2012) Methods of concentrating stallion semen. Journal of Equine Veterinary Science 32(8): 424-429.
- Aurich JE (2012) Artificial insemination in horses-more than a century of practice and research. Journal of Equine Veterinary Science 32(8): 458-463.
- Henkel RR, Schill WB (2003) Sperm preparation for ART. Reprod Biol Endocrinol 1: 108.
- Loomis PR (2006) Advanced methods for handling and preparation of stallion semen. Vet Clin North Am Equine Pract 22(3): 663-676.
- Ball BA, Vo AT, Baumber J et al. (2001) Generation of reactive oxygen species by equine spermatozoa. Am J Vet Res 62(4): 508-515.
- Yelumalai S, Kashir J, Jones C, Bagheri H, Lin Oo S, et al. (2012) Clinician induced (iatrogenic) damage incurred during human infertility treatment: detrimental effects of sperm selection methods and cryopreservation upon the viability, DNA integrity, and function of human sperm. Asian Pacific Journal of Reproduction 1(1): 69-75.
© 2019 Gustavo R Larentis. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






