- Submissions

Full Text
COJ Technical & Scientific Research
Water and Life, Both Animal and Vegetal
Emilia Fisicaro, Carlotta Compari and Antonio B*
Department of Food and Drug, University of Parma, Italy
*Corresponding author:Antonio Braibanti, Department of Food and Drug, Biological Physical Chemistry Section, University of Parma, Italy
Submission: November 22, 2022; Published: January 17, 2023

Volume4 Issue1January , 2023
Introduction
The existence of the thermodynamic potential μsolv for Implicit Solvent is bound to the existence of a layer of molecular arrangements (icebergs), typical of water when behaving as Implicit Solvent, The Icebergs formed when a gas or other molecular unit, even of size of a protein, enters in the Implicit Solvent water. The icebergs react again with the Implicit Solvent at constant thermodynamic potential μsolv. Completing the complex iceberg around the entering unit. This interpretation of the structure of water as Implicit Solvent is new, as far as we know, and very profitable. This study is a development of the paper recently published where we had performed a statistical analysis of a set of eighty-one compounds, according to the rules of Chemometric Analysis of Variance. That analysis had leaded us to conclude that, notwithstanding the remarkable difference between one another group in structure, in molecular size, in aggregation state, the motive functions of all the groups, show similar properties. The new paper is concerning the equilibrium constants of Chemical and Biological Hydrophobic Hydration Processes. In the new manuscript, we are developing the table of a previous paper, containing thermal equivalent dilution. This name, attributed by us to this table, is not satisfactory (the name is not satisfactory, but the reported numbers are correct) and we are proposing to call that table as Ergodic Algorithmic Model for Symmetrical Hyperbolas. We show, in this new paper, that every determination of equilibrium constant examined in this Laboratory is concerning only with compounds presenting, in their partition function, Symmetrical Hyperbolas. This result is an important general conclusion, that we hope will be appreciated and used by many researchers [Figure 1].
Figure 1:Iceberg Formation, if a molecular unit enters in water, that behaves as Implicit Solvent.
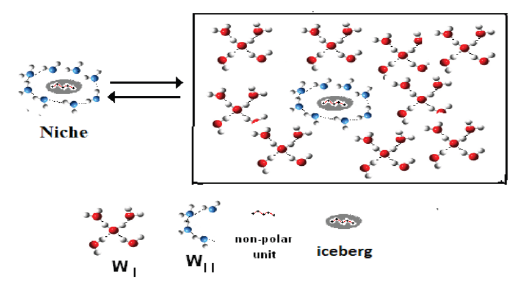
We demonstrate also that the out-of-diagonal point A or B or C is Inexistent. We mean
Inexistent Point, at least for the validated chemo-metric lot of our compounds, because this
lot is referred exclusively, at 100% probability, to compounds with
symmetrical hyperbolas, with exclusion of any other molecular
arrangement. The results of the chemometric statistical analysis of
errors renders our results as having the strength of a general law.
The paper will be composed of three parts, distinct but logically
connected to one another.
a) In the first part, we are showing how the introduction
of the character of Implicit Solvent for water, guarantees the
introduction in the system of a separated phase at constant
thermodynamic potential μSolv, thus giving rise, for the implicit
solvent water, to a layer with the character of a separate phase,
at constant Thermodynamic Potential, μSolv.
b) The property of forming a “separate phase” depends on
the capacity of water, or better on that part of water which is
at constant Thermodynamic Potential, μSolv of giving rise to
living systems either animal or vegetal. Only water, as a separate
phase with the property of implicit solvent, has the capacity of
transmitting life. both vegetal and animal life. We are calling
your attention on this point with the purpose of helping you in
your choices of molecular events.
c) In the third part of the paper, we will show how the
different types of water complexes (WII) are reflected in the
structures of icebergs, hydrated complexes around molecular
units (gas or solid) entering in the solution. Only symmetrical
hyperbolic functions have been found in the hole set, statistically
validated, of compounds studied in this laboratory.
We note that Implicit solvent is formed whenever the solvent is in excess of any other component, and its properties are no more dependent of its concentration.
© 2023 Antonio B. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






