- Submissions

Full Text
COJ Technical & Scientific Research
Investigation the Existence of H2A Gene in Leishmaniatropica
Marwa Alhabash1 and Mohammad Maarouf*2
1Department of Biochemistry, Faculty of Pharmacy, Damascus University, Syria
2Department of Microbiology, Faculty of Pharmacy, Damascus University, Syria
*Corresponding author: Mohammad Maarouf, Department of Microbiology, Faculty of Pharmacy, Damascus University, Damascus, Syria
Submission: March 13, 2020; Published: April 23, 2020

Volume2 Issue5April, 2020
Abstract
Cutaneous Leishmaniasis caused by Leishmaniatropica is one of the most important health issues in Syria which exacerbated after the war in Syria. Reduction of the high prevalence of Leishmanias is in Syria requires the availability of an effective vaccine. DNA vaccine has recently provided a promising new approach. Nucleosomal core histones are very important for the parasites life, their genes showed a protective effect as DNA vaccines against infections by many species of Leishmania. The existence of these genes, like H2A gene, was not proved in L. tropica. Our study aimed to investigate the existence of H2A gene in a Syrian strain of L. tropica promastigotes and their expression. We isolated DNA and RNA from these parasites, synthesized cDNA, designed the necessary primers for gene`s amplification, and standardized the PCR conditions. This is the first study that proved the existence of this gene in a Syrian strain of L. tropica promastigotes. The sequence of the cDNA H2A gene was defined and published in Gene bank, its size was 399bp. Expression was also proved at the level of cDNA.
Keywords: Leishmaniatropica; H2A gene; Syria
Abbreviations: cDNA: complementary deoxy ribonucleic acid, DNA: deoxy ribonucleic acid, RNA: ribonucleic acid, L.: Leishmania.
Introduction
Leishmaniases are infectious diseases caused by several species of the intracellular protozoan of Leishmania, which are transmitted by sandfly [1]. Leishmaniasis has different clinical manifestations, ranging from self-healing cutaneous ulcers to potentially fatal visceral infection [1]. These diseases represent a global public health problem, with an estimated prevalence of 12 million human beings infected. Syria is one of 9 countries have the majority of cutaneous Leishmaniasis cases in the world [2]. Current control is based on chemotherapeutic treatments which are expensive, toxic and associated with high relapse and resistance rates. Vaccination remains the best hope for control of all forms of the disease, so the development of a safe, effective and affordable anti Leishmanial vaccine is a critical global public-health priority. Immunization with naked DNA is a relatively new approach, which promises to revolutionize the prevention and treatment of infectious diseases [3]. Many studies have been made to find a protective antigen against Leishmaniasis and many candidates have been studied forth is aim, including histones. Histones are structural proteins found in the nucleus, where they play an important role in the organization and function of chromatin [4]. These highly-conserved proteins that elicit strong immune responses are generally designated as pan antigens [5]. Specific antibodies for parasite histones, obtained from dogs with visceral Leishmaniasis, react against Leishmania H2A [6], H3 [7], H2B and H4 [8] but do not recognize mammalian histones. In the right conditions,these panantigens can provide the immune modulatory properties needed for vaccine design [9]. So these histones and their genes are good candidates for vaccine.H2A gene was sequenced in many species of Leshmania, but not in L. tropica, and their immunogenicity was tested [10,11]. We chose H2A gene, to design a DNA vaccine against the infection with Leishmaniatropica which is responsible of the most cutaneous cases in Syria. The existence of H2A gene wasn’t proved in L. trpoica. In this study; A methodical study was first made to know if this gene exists in the genome of a Syrian strain of Leishmania tropica, to prove if there is an expression of H2A Gene in these parasites, and to sequence this gene.
Materials and Methods
Primer design for PCR
Sequence of H2Agene in Leishmaniatropica is not known so we made an alignment for the sequences of this gene in other species using CLC free workbench 7 and Vector NTI Express programs and theclustalw2 and emboss_needle tools. Primers were designed using http://www.idtdna.com/calc/analyzer and http://www.geneinfinity.org/sms/sms_primanalysis.html tools. High purified Primers were ordered from VBC- Biotech, Vienna. Final sequences for primers were: [forward, 5’-ATTAGAATTC ATG GCT ACTCCTCGCAGCG-3’; reverse, 5’-CT TCTAGA CTAAGCGCTCGGTGTCGCCTTG-3’]. Underlined are the ECORI and XBAI restriction sites included for direct cloning in the PCI mammalian expression vector [Promega, United States].
DNA extraction
DNA was extracted from the promastiogotes of a Syrian strain Leishmaniatropica [LCEBS,Damascus, Syria]. DNA was isolated from Leishmania promastigotes, cultured on RPMI-1640 medium [Lonza, Switzerland] supported with Fetal bovine serum [Cytogen, GmbH, Germany], with DNA extraction kit [Thermoscientific, Lithuania] according to manufacturer’s instructions.
RNA isolation and cDNA synthesis
Total RNA was extracted from harvested Leishmaniatropica promastigotes using TRI reagent [Sigma, St Louis, MO, USA]. RNA was quantified by NanoDrop1000 [Thermo Fisher Scientific MA, USA]. RNA-integrity was checked by separating in 1.5% agarose gel. First strand cDNAs were synthesized using Revert Aid First Strand cDNA Synthesis kit [Thermoscientific, Lithuania] according to manufacturer’s instructions.
Polymerase chain reaction
DNA and cDNA of H2A gene were amplified by thermal cycler [Bio-Rad, USA] using PCR master mix [Thermo scientific, Lithuania] and the primers mentioned above. PCR protocol was optimized using gradient annealing temperatures. Reactions were performed in 25 μl of a mixture containing 1μl of 10μM from each primer, 2, 5 μl of template DNA or cDNA [110 ng\μl], 12, 5 μl of PCR master mix and 8 μl of PCR water. Thermal cycling conditions were 95°C for 5min followed by 35 cycles of 95°C for 1min, 61.6°C for 45sec and 72°C for 1 min finally 72°C for 5 min. PCR products were loaded on a 2% agarose gel, stained with ethidium bromide, and visualized on a UV transilluminator.
Plasmid construction and purification
PCR product containing H2Acoding sequence was purified using Gene Jet PCR Purification kit [Thermoscientific, Lithuania] according to manufacturer’s instructions. Purified cDNA and the PCI mammalian expression vector [Promega, United States] were double digested with ECORI plus XBAI. Digested cDNA was ligated with the digested PCI mammalian expression vector using t4 DNA ligase [Thermoscientific, Lithuania]. PCI-H2A clones were transformed in to E-coli TOP 10. Recombinant plasmid was extracted from colonies and purified using GF-1 plasmid DNA extraction kit [Vivantis, Malaysia].
Sequences Analysis
Full-length double-stranded sequence analysis was performed on purified Recombinant plasmid PCI-H2Aby sequencing, using T7 [forward] and PCR 3’[reverse] vector primers on automated sequencers [ABI PRISM BigDye Terminator Cycle Sequencing Ready Reaction Kit, Perkin-Elmer, Foster City,USA].
Results and Discussion
Evaluation DNA and RNA quality and purity
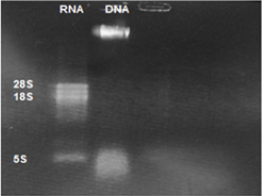
Extracted DNA electrophoresison a 1% agarose gel only showed one band which proves that there isn’t any degradation in the extracted DNA [Figure 1]. The purity of the DNA was 1.8 which showed a high degree of purity. Extracted RNA electrophoresis on a 1.5% agarose gel showed a standard profile and there was no contamination with genomic DNA [Figure 2]. The purity of the RNA was 2.1 which showed a sufficient degree of purity.
Figure 1: Electrophoresis of extracted Leishmaniatropica DNA: Lane 1 DNA ladder 1Kb, Lane 2,3 extracted Leishmaniatropica DNA.
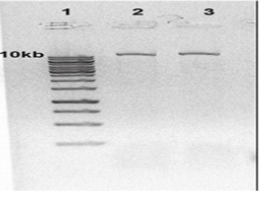
Figure 2: Electrophoresis of extracted Leishmaniatropica RNA: Lane1 Leishmaniatropica RNA, Lane 2 extracted Leishmaniatropica DNA.
Polymerase chain reaction
PCR protocol was optimized using gradient annealing temperatures and we chose the most suitable annealing temperature which was 61.6°C.Gel electrophoresis of H2APCR product on the level of DNA and cDNA showed only one bandabout400 base pair which proves the specificity of our primers. So we proved the existence of H2A gene in the genome of a Syrian strain of Leishmaniatropica [Figure 3] and in the cDNA of this strain [Figure 4].
Figure 3: Amplification products of Leishmaniatropica H2Aon 2% agarose gel electrophoresis stained with ethidium bromide. Lane 1 DNA ladder 50bp, lane 2 H2A- DNA amplification product about 400bp.
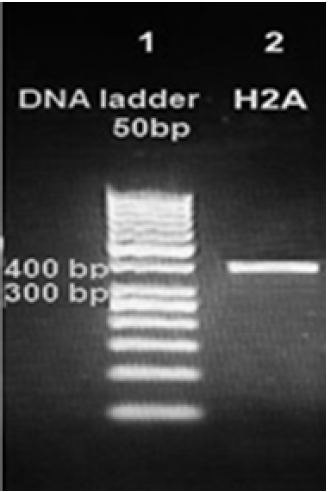
Figure 4: Amplification products of Leishmaniatropica H2A on 2% agarose gel electrophoresis stained with ethidium bromide. Lane 1 DNA ladder 50bp, lane 2 H2A-cDNA amplification product about 399 bp.

Cloning and sequencing of the H2A cDNA
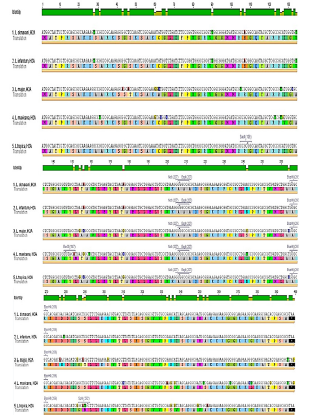
In an effort to clone and characterize the Leishmaniatropica H2A gene, a cDNA clone was identified and sequenced. The sequence was submitted to the Gene bank underKT956066.1 Accession number. The complete nucleotide sequence of the 399bp cDNA insert, and the deduced amino acid sequence in comparison with other species were showed in [Figures 5 & 6] respectively. The deduced amino acid sequence predicts a protein containing 132 amino acids. The H2A predicted amino acid sequence was compared with the sequences of proteins coded by cDNA of H2A in other species of Leishmania such as L.infantum, L.major, L.donovania, L.mexicana and L.braziliensis using Vector NTI Program. This search revealed that H2A belongs to the histone H2A family. Interestingly, L.tropicaH2A shows significant similarity with H2A histones of L.infantum [92.4%], L.major [91.7%], L.donovania [92.4%], L.mexicana [91.7%] and L.braziliensis [78%]. The last high similarity shows that a good H2A DNA vaccine could protect from infection by the different species of Leishmania.
Figure 5: The complete nucleotide sequence of the H2A cDNA.

Figure 6: Nucleotide sequences of H2A cDNA for L.tropica, L.donovani, L.infantum, L.major and L.mexicana. The deduced amino acid sequence is indicated below the cDNA sequence.
Conclusion
Our results proved the existence of H2A gene in the genomic DNA and the cDNA of a Syrian Leishmaniatropica strain. We proved for the first time the existence of H2A gene in Leishmaniatropica, and defined the sequence of the cDNA of this gene and submitted it to the Gene bank under KT956067 accession number. PCI-H2A clones could now be tested to evaluate their immunogenicity as DNA vaccine against Leishmania species.
References
- Desjeux P (2004) Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis 27(5): 305-18.
- WHO | Burden and distribution. World Health Organization.
- Mutiso JM (2013) Development of Leishmania vaccines: predicting the future from past and present experience. J Biomed Res 27(2): 85-102.
- Carneiro MW, Santos DM, Fukutani KF, Clarencio J, Miranda JC, Brodskyn C, et al. (2012) Vaccination with infantum chagasi Nucleosomal Histones Confers Protection against New World Cutaneous Leishmaniasis Caused by Leishmania braziliensis. PLoS One 7 (12): e52296.
- Requena JM, Alonso C, Soto M, Requena JM, Alonso C, Soto M (2000) Evolutionarily conserved proteins as prominent immunogens during Leishmania infections. Parasitol Today 16(6): 246-250.
- Soto M, Requena JM, Quijada L, García M, Guzman F, Patarroyo ME, et al. (1995) Mapping of the linear antigenic determinants from the Leishmania infantum histone H2A recognized by sera from dogs with leishmaniasis. Immunol Lett 48(3): 209-214.
- Soto M, Requena JM, Quijada L, Gomez LC, Guzman F, Patarroyo ME, et al. (1996) Characterization of the antigenic determinants of the Leishmania infantum histone H3 recognized by antibodies elicited during canine visceral leishmaniasis. Clin Exp Immunol 106(3): 454-61.
- Soto M, Requena JM, Quijada L, Perez MJ, Nieto CG, Guzman F, et al. (1999) Antigenicity of the Leishmania infantum histones H2B and H4 during canine viscerocutaneous leishmaniasis. Clin Exp Immunol 115(2): 342-349.
- Carrión J, Folgueira C, Alonso C. (2009) Development of immunization strategies against leishmaniosis based on the leishmania histones pathoantigens 1(1): 101-3.
- Iborra S, Soto M, Carrión J, Alonso C, Requena JM. (2004) Vaccination with a plasmid DNA cocktail encoding the nucleosomal histones of Leishmania confers protection against murine cutaneous leishmaniosis. Vaccine 22(29-30): 3865-76.
- Carrión J, Folgueira C, Alonso C. (2008) Transitory or long-lasting immunity to Leishmania major infection: the result of immunogenicity and multicomponent properties of histone DNA vaccines. Vaccine 26(9): 1155-65.
© 2020 Mohammad Maarouf. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






