- Submissions

Full Text
COJ Technical & Scientific Research
Significance of Functional Group in Indole Molecule: Toward Sensitivity of Biosensor
Md. Zaved Hossain Khan*
Jashore Science and Technology University, Bangladesh
*Corresponding author: Md. Zaved Hossain Khan, Jashore Science and Technology University, Jashore, Bangladesh
Submission: January 03, 2020; Published: January 22, 2020

Volume2 Issue4January, 2020
Abstract
In this opinion, I focus my attentions on the effect of the functional group in indole molecules onto their potential response with disuccinimidyl suberate (DSS) modified indium tin oxide (ITO) electrode. It was aimed to discuss the variation of potential response of self-assembled monolayer modified ITO electrode for various molecules containing indole ring. A great response with different sensitivity was demonstrated to achieve a linear relationship with the different analyte concentration. However, the magnitude of the potential shift was found to depend on the molecular structure. From a fundamental viewpoint, it seems that interest is based not only on high sensitivity but also on the effect of the functional group substituted onto the indole ring.
Keywords: Modified electrode; Potential response; Sensitivity; Biomolecules; Functional group
Introduction
Figure 1:SAM modified electrode for biomolecule detection.
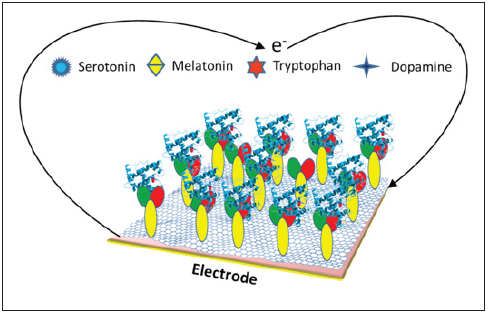
Detection of biomolecules is of great interest in the view of biological and medical science from last few decades. Different types of modified electrodes have been used as amperometric/potentiometric sensors for this purpose of detection (Figure 1). Recently, we found that the successive modification of an ITO electrode with aminopropyltriethoxysilane (APTES) and disuccinimidyl suberate (DSS) is useful for the potentiometric sensing of molecules containing an indole ring [1-3]. A commercial development of biosensors for industrial applications requires their optimization in terms of the stability and sensitivity [4]. The sensitivity of the biosensor is very important for practical applications. The sensitivity depends not only upon the adsorption on the surface but also upon the bioactivity, immobilization, and interaction conditions etc. We focus our attention on the structure of the indole molecule as one of the factors affecting the magnitude of potential shift. Several researchers have reported the effect of the functional group on the electrochemical behaviour of indole molecules [5-7]. We showed that the enhancement of the potential shift depended on the substituted group that was introduced into the indole ring. Molecules containing the 5-hydroxy group on their indole ring have an enhanced potential shift [1]. It is well established that the transport properties of a molecule to electrode depend on the intrinsic properties of the molecule, such as the energy gap between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO), the molecular conformation, and the molecular bond characteristics (e.g., saturated versus unsaturated). In addition, the chemical groups anchoring the molecule to the electrode, the work function of the electrode, and the contact geometry of the moleculeelectrode interface [8,9]. From our experiment it was clear that the enhanced potential shift with 5-hydroxy group, such as serotonin (5HT), 5-hydroxyindoleaceticacid (5-HIAA), and 5-hydroxy tryptophan (5-HTP) is stronger than that of with 5-methoxy group, such as melatonin (MT). Large potential shift might be obtained for 5-hydroxy indoles during measurement concerning their redox reaction that enhanced the sensitivity during potentiometric measurement.
Significance of functional group
Table 1:Analytical parameters for 5-HT, 5-HTP, 5-HIAA, and MT measurement. The sensitivity was calculated from the slope (correlation coefficient was 0.995 in all cases).
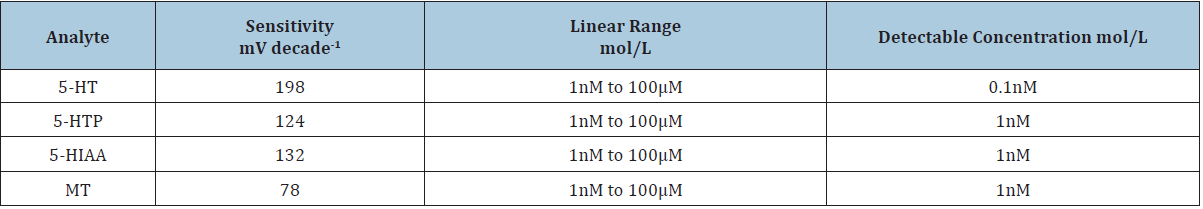
Previously we have reported that DSS treated ITO can detect
several biomolecules. (Table 1) shows the sensitivity and detection
limit of 5HT, 5-HIAA, MT, and 5-HTP with DSS modified ITO
electrode. A high sensitivity of 198mV decade-1 with a minimum
detectable concentration of 0.1nM was observed for 5-HT, whereas
for 5-HTP, 5-HIAA, and MT, the sensitivity was 124, 132, and 78mV
decade-1, respectively. Even though 5-HT, 5-HTP, and 5-HIAA are
redox active molecules bearing the 5-hydroxy group in their indole
ring, the highest sensitivity and lowest detection limit was observed
for 5-HT.
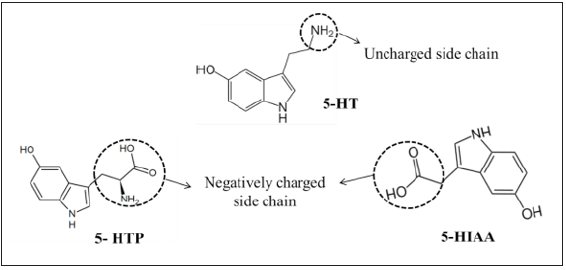
A possible explanation can be given by focusing attention on
the side chain on C3 position of indole ring. 5-HT has an uncharged
side chain, whereas 5-HTP and 5-HIAA have a negatively charged
carboxylic group in the side chain (Figure 2). The redox-active
moiety in the compounds and the radicals is the indole ring whose
electronic structure may affect by the electron-donating or electron
withdrawing group substituted in the C3 position. The influence of
the charge of the side chain of 5-hydroxyindole derivatives on the
kinetics of their reactions with charged substrates might affect the
magnitude of the potential shift in electrochemical measurement
[10]. As a result, we observed higher potential shift for 5-HT,
comparing with 5-HTP and 5-HIAA.
Figure 2:Structure of 5-HT, 5-HIAA, and 5-HTP.
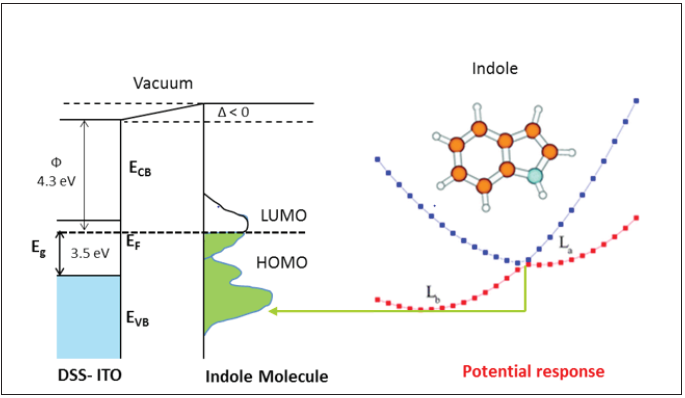
For the efficient charge transfer, the relative position of donor LUMO and acceptor LUMO is crucial [11]. For the efficient charge transfers from donor to acceptor component, effective charge transport are important parameters for different potential response mechanism elucidation (Figure 3). To estimate the relative position of HOMO/LUMO levels, valuable information can be given by electrochemical data [8,11]. During potentiometric measurement the knowledge of these levels is required to find the donoracceptor behavior. The values of energy levels of LUMO and HOMO and energy band gap estimated from the electrochemical data can be an explanation of the effect of function group on the potential shift. 5-hydroxy indoles have a high electron density at the HOMO level [11]. When the LUMO level shifts towards downwards as the acceptor group in the backbone increases, then the electron affinity increases as the number of acceptor group increases [8], thereby we might observed enhance potential shift for 5-hydroxy indoles. But the highest response was observed for 5-HT with additional uncharged side chain onto the indole ring. This indicates that the decrease in LUMO is more as compared to that of the HOMO. In the case of melatonin, the formerly HOMO-1 in indole introduced by methoxy substituent as an additional node in the molecular, and which becomes the HOMO in 5-methoxyindole. By this way the energy ordering between indole, 5-methoxyindole and melatonin are changes for the HOMO and HOMO-1 [9]. Hence, the energy mismatch of LUMO levels will be more than the mismatch of HOMO levels at the interface [9,12].
Figure 3:Equilibration of chemical potential leads to a charge transfer.
Concluding remarks and recommendation
The contribution of redox processes and functional group attached to the indole ring to the potential response of the electrode seems important from the above discussion. 5-hydroxyindole with uncharged side chain shows higher response than those has a negatively charged group in the side chain. It is evident that the enhanced potential shift is closely related to the functional group substituted onto the indole ring. Details mechanism considering the electronic structure of indoles can be a future target investigation. A details density functional theory (DFT) calculation might be effective to understand density of energy states of different analyte and their response behavior.
References
- Khan MZH, Nakanishi T, Osaka T (2011) Potentiometric detection of serotonin, melatonin, and their precursors/metabolites with monolayer-modified indium tin oxide electrode and their concentration dependency. Sensor Letters 9(5): 1849-1852.
- Khan MZH, Nakanishi T, Kuroiwa S, Hoshi Y, Osaka T (2011) Effect of surface roughness and surface modification of indium tin oxide electrode on its potential response to tryptophan. Electrochimia Acta 56(24): 8657-8661.
- Nakanishi T, Ueno T, Matsunaga M, Khan MZH, Osaka T (2010) Potential response of monolayer-modified indium tin oxide electrodes to indole compounds. Electroanalysis 22(4): 393-398.
- Khan MZH (2016) Effect of ITO surface properties on SAM modification: A review toward biosensor application. Cogent Engineering 3(1): 1170097.
- Wu X, Huang F, Duan J, Chen G (2005) Electro chemiluminescent behavior of melatonin and its important derivatives in the presence of Ru(bpy) (3) (2+). Talanta 65(5): 1279-1285.
- Süzen S, Demírcígíl BT, Buyukbingol E, Özkan SS (2003) Electroanalytical evaluation and determination of 5-(3-indolyl)-2-thiohydantoin derivatives by voltammetric studies: Possible relevance to in vitro metabolism. New J Chem 27(6): 1007-1011.
- Nematollahi D, Dehdashtian S, Niazi A (2008) Electrochemical oxidation of some dihydroxybenzene derivatives in the presence of indole. J Electroanal Chem 616(1-2): 79-86.
- Li XL, Jin H, Hihath J, Bingqian XQ, Lindsay SM, et al. (2006) Conductance of single alkanedithiols: conduction mechanism and effect of molecule-electrode contacts. J Am Chem Soc 128(6): 2135-2141.
- Stadler R, Thygesen KS, Jacobsen KW (2005) Forces and conductance in a single - molecule bipyridine junction. Phys Rev B 72(24): 241-401.
- Jovanovic SV, Steen S, Simic MG (1990) One-electron reduction potentials of 5-indoxyl radicals: a pulse radiolysis and laser photolysis study. J Phys Chem 94(9): 3583-3588.
- John YT, Brand CV, Wollenhaupt M, Pratt DW, Meerts WL, et al. (2011) Rotationally resolved electronic spectroscopy of biomolecules in the gas phase Melatonin. Journal of Molecular Spectroscopy 268(1-2): 115-122.
- Catalan J, Perez P, Acuna A (1986) Indole spectroscopy: The location of the 1La and 1Lb electronic states and the absorption spectrum. Journal of Molecular Structure 142: 179-182.
© 2020 Md. Zaved Hossain Khan. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






