- Submissions

Full Text
COJ Technical & Scientific Research
How the Puzzles of the Transurethral Resection of the Prostate (TURP) Syndrome and the Adult Respiratory Distress Syndrome (ARDS) were Resolved? Discovering Volumetric Overload Shocks
Ahmed N Ghanem*
Consultant Urological Surgeon, Egypt
*Corresponding author:Ahmed N Ghanem, Consultant Urological Surgeon, No 1, Jasmine Tower, President Mubarak Street, Mansoura 35511, Egypt
Submission: October 11, 2018;Published: October 15, 2018

Volume1 Issue2 October 2018
Opinion
The last 32 years of my career life were spent in investigating and reporting these articles [1-5]. The articles recognizes 2 new types of shocks and its treatment, proves that Starling’s law for the capillary interstitial fluid transfer is wrong and provides an alternative mechanism; the hydrodynamics of a porous orifice (G) Tube. These discoveries resolve the puzzles of 2 clinical syndromes discovering its patho-etiology and new successful treatments; namely the transurethral resection of the prostate (TURP) syndrome and the adult respiratory distress syndrome (ARDS).
Volumetric Overload Shock (VOS) is a condition caused by massive fluid infusions in a short time and is of two types; Type one (VOS1) and Type two (VOS2). VOS1 is induced by sodium-free fluid gain of 3.5-5 liters in one hour such as Glycine, Glucose, Mannitol and Sorbitol. It is known as the TURP syndrome [5] or hyponatraemic shock [6]. VOS2 is induced by massive infusion of sodium-based fluids such as normal saline, Ringer, Hartmann, plasma, plasma substitutes and blood transfusions that may complicate the therapy of VOS1. VOS2 also complicates fluid therapy in critically ill patients suffering from other known shocks such as hypovolaemic, haemorrhagic and septic shocks and presents with ARDS [7]. VOS2 is induced by the gain of 12-14 liters of sodium-based fluids when reported in ARDS. The occurrence of massive interstitial tissue oedema with congestion of vital organs, pleural and peritoneal effusions, in the presence of hypotension shock, casted doubt on Starling’s law! These issues were investigated at the clinical and physiological/physical fronts.
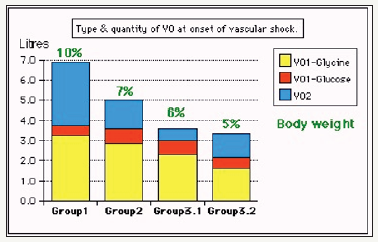
Two clinical studies aiming to understand the TURP syndrome and recognizing VOS were done. A prospective clinical study on 100 consecutive TURP patients of whom the condition of TURP syndrome affected 10 patients with severe hypotension and bradycardia and severe acute dilution hyponatraemia of < 120 mmol/l [5]. Volumetric overload was the only significant factor in causing the condition. The second clinical study involved a case series of 23 cases of the TURP syndrome manifesting as VOS1. Volumetric overload quantity and type is shown in (Figure 1). The first 3 cases died as they were diagnosed and treated erroneously as one of the recognized shocks and treated with further volume expansion. The remaining 20 patients were correctly diagnosed as VOS1 and treated with hypertonic sodium therapy (HST) of 5% Sodium Chloride or 8.4% Sodium Bicarbonate. Each patient passed 4-5 litres of urine followed by recovery from shock and coma. This treatment was successful in curing all patients bringing them back from dead [4].
Figure 1:shows volumetric overload (VO) quantity (in liters and as percent of body weight) and types of fluids. Group 1 was the 3 patients who died in the case series as they were misdiagnosed as one of the previously known shocks and treated with further volume expansion. Group 2 were 10 patients from the series who were correctly diagnosed as volumetric overload shock and treated with hypertonic sodium therapy (HST). Group 3 were 10 patients who were seen in the prospective study and subdivided into 2 groups; Group 3.1 of 5 patients treated with HST and Group 3.2 of 5 patients who were treated with guarded volume expansion using isotonic saline.
The physical investigation involved studies of the hydrodynamics of the porous orifice (G) tube comparing it to that of Poiseuille’s tube. Thousands of experimental measurements of pressures at various parts of a circulatory system incorporating the G tube in a chamber to mimic the capillary-interstitial fluid compartment. The effect of changing the proximal (arterial), the distal (venous) pressures and the diameter of the inlet on side pressure of the G tube and chamber pressure as well as the dynamic magnetic field like fluid circulation around the G tube. It is quite remarkable how this circulatory model mimics the circulatory system in heath and disease. This dynamic magnetic field like fluid circulation around the G tube and surrounding it in C chamber provides adequate replacement for Starling’s law [3]. The physiological equivalent of this physical study was done on the hind limbs of sheep. It demonstrated that arterial pressure causes suction not filtration due to the effect of pre- capillary sphincter. Venous pressure augmented filtration and oedema or dropsy formation.
Shock is a disturbance at the capillary cellular level impairing the capillary-interstitial fluid transfer; hindering delivery of oxygen and removal of waste products. The process is governed by Starling’s law [8]. In this law the arterial pressure is considered the force causing capillary filtration! If this is true, how come that arterial hypertension though very common never causes oedema? Starling based his hypothesis on Poiseuille work on strait uniform brass tubes. Latter evidence however demonstrated that the capillary is a porous narrow orifice (G) tube as it has a pre-capillary sphincter [9] and pores that allow the passage of plasma proteins [10]. As the capillary pores allow the passage of plasma molecules, nullifying the osmotic pressure of plasma proteins i.e oncotic pressure does not exist, a call for reconsideration of Starling’s hypothesis was previously made but there was no alternative at that time [11]. This replacement came to light when the hydrodynamics of the G tube were discovered.
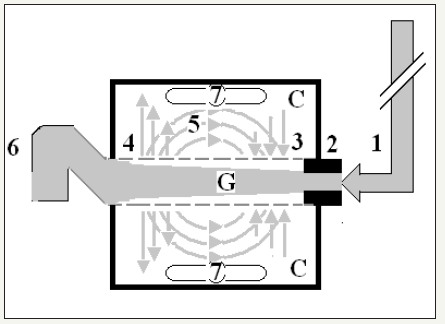
The hydrodynamics of the G tube [3,12], (Figure 2) demonstrated that the proximal (arterial) pressure induces a negative side pressure gradient on the wall of the G tube causing suction most prominent over the proximal half and turns into positive pressure over the distal half. Incorporating the G tube in a chamber (C), representing the interstitial space surrounding a capillary, demonstrated a rapid dynamic magnetic field-like fluid circulation between the C and G tube lumen. This is a mixing engine between C and G effecting rapid irrigation under negative pressure i.e. without flooding or oedema or dropsy formation. Incorporating the G tube and C in a circulatory model driven by electric pump inducing proximal pressure similar to arterial pressure; causing suction from C into the lumen of G tube. The distal (venous) pressure augments filtration. This proves that the arterial pressure causes suction not filtration at the capillary interstitial fluid circulation, and hence Starling’s law is wrong. The reported hydrodynamics of the G tube provides an adequate mechanism for the capillary interstitial fluid circulation. This illustrates how 2 new types of vascular shocks and a replacement of Starling’s law were discovered.
Figure 2:Diagram of the porous orifice (G) tube enclosed in chamber (C) based on several photographs demonstrating the magnetic field-like G-C circulation phenomenon. The proximal inflow (arterial) pressure (1) pushes fluid through the orifice (2) creating fluid jet in the lumen of the G tube. The fluid jet creates negative side pressure gradient causing suction maximal over the proximal half of the G tube near the inlet (3) that sucks fluid into lumen. The side pressure gradient turns positive pushing fluid out of lumen over the distal half maximally near the outlet (4). Thus, the fluid around G tube inside C moves in magnetic field-like fluid circulation (5) taking an opposite direction to lumen flow of G. tube. The inflow (arterial) pressure (1) and orifice (2) induce the negative side pressure energy creating the dynamic G-C circulation phenomenon that is rapid, autonomous and efficient in moving fluid out from the G tube lumen at (4), irrigating C at (5), then sucking it back again at (3), maintaining net negative energy pressure (7) inside C. The distal outflow (venous) pressure (6) enhances outflow at (4) and its elevation may turn the negative energy pressure (7) inside C into positive, increasing volume and pressure inside C chamber.
References
- Ghanem AN, Ghanem SA (2016) Volumetric overload shocks: Why is starling’s law for capillary interstitial fluid transfer wrong? The hydrodynamics of a porous orifice tube as alternative. Surgical Science 7(6): 245-249.
- Pindoria N, Ghanem SA, Ghanem KA, Ghanem AN (2017) Volumetric overload shocks in the patho-etiology of the transurethral resection prostatectomy syndrome and acute dilution hyponatraemia. Integr Mol Med.
- Ghanem KA, Ghanem AN (2017) The proof and reasons that Starling’s law for the capillary-interstitial fluid transfer is wrong, advancing the hydrodynamics of a porous orifice (G) tube as the real mechanism. Blood, Heart and Circ 1(1): 1-7.
- Ghanem KA, Ghanem AN (2017) Volumetric overload shocks in the patho-etiology of the transurethral resection prostatectomy syndrome and acute dilution Hyponatraemia: The clinical evidence based on 23 case series. Basic Research Journal of Medicine and Clinical Sciences Vol. 6(4).
- Ghanem AN, Ward JP (1990) Osmotic and metabolic sequelae of volumetric overload in relation to the TURP syndrome. Br J Uro 66(1): 71-78.
- Harrison RH, Boren JS, Robinson JR (1956) Dilutional hyponatraemic shock: another concept of the transurethral prostatic reaction. J Urol 75(1): 95-110.
- Ashbaugh DG, Bigelow DB, Petty TL, Levine BE (1967) Acute respiratory distress in adults. Lancet 2(7511): 319-323.
- Starling EH (1886) Factors involved in the causation of dropsy. Lancet 2: 1266-1270, 1330-1334 and 1406-1410.
- Rhodin JA (1967) The ultra-structure of mammalian arterioles and precapillary sphincters. J Ultrastructure Research 18(1-2): 181-222.
- Karnovesky MJ (1967) The ultra-structural basis of capillary permeability studied with peroxidase as a tracer. J Cell Biol 35(1): 213- 236.
- Renkin EM (1986) Some consequences of capillary permeability to macromolecules: Starling’s hypothesis reconsidered. Am J Physiol (Heart Circ Physiol) 250(5): H706–H710.
- Ghanem AN (2001) Magnetic field-like fluid circulation of a porous orifice tube and relevance to the capillary-interstitial fluid circulation: Preliminary report. Medical Hypotheses 56(3): 325-334.
© 2018 Ahmed N Ghanem. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






