- Submissions

Full Text
COJ Technical & Scientific Research
Homogenization Medium Effect on Permittivity for Barium Titanate Ceramics
Hatice Zehra Dolek*
Department of Physics, Mustafa Kemal University, Turkey
*Corresponding author: Hatice Zehra Dolek, Department of Physics, Mustafa Kemal University, Antakya, Hatay, Turkey
Submission: August 17, 2018;;Published: September 10, 2018

Volume1 Issue1 July 2018
Abstract
Effect of the homogenization medium on the dielectric properties of BaTiO3 ceramics were investigated by solid-state reaction using distilled water, methanol, and acetone. Homogenization medium dramatically changed the microstructure of BaTiO3 ceramics resulting in significant effect on electrical properties. Depending on the homogenization medium, the sinterization process led directly to normal and/or abnormal grain growth of the samples. The structure and morphologic properties of BaTiO3 ceramics were characterized using X-ray Diffraction (XRD) and Scanning Electron Microscopy (SEM). The complex permittivity and complex electric modulus of the ceramics were studied in a wide frequency range of 100μHz-20MHz at room temperature. BaTiO3 ceramics have a relaxation time with different mean time constant called Maxwell-Wagner-Sillars (MWS) type relaxation. Results of permittivity and resistivity measurements showed that using water instead of acetone and methanol have a positive effect on electrical properties of barium titanite ceramics with small dielectric loss.
keywords Homogenization medium; Agglomeration/agglomerates; Barium titanite; Grain growth; Dielectric materials/properties
Introduction
Barium Titanite (BaTiO3) has been commonly employed in as electronic components like capacitor, thermistor, varistor due to its outstanding dielectric, piezoelectric and ferroelectric properties [1- 7]. BaTiO3 can be produced from different synthesis methods such as solid-state reaction, sol precipitation, and direct synthesis [8-10]. BaTiO3 powders with submicron particles are usually synthesized in wet chemical processes and the predominant domain structure is the cubic phase. The cubic phase of BaTiO3 ceramics is paraelectric and its dielectric properties are poor [4-11]. The tetragonal phase of the BaTiO3 have decreased with decreasing particle size due to size effects and hydroxyl ion effects in wet chemical synthesis [10-13]. The relative volume fraction of cubic and tetragonal phases in the powders prepared by hydro-thermal techniques was found to be very sensitive to synthesis condition [14]. It is reported that particle sizes of powders increase with increasing water and crystal structure of particle change from tetragonal to cubic in powders prepared by low-temperature alkoxide-hydroxide precipitation synthesis [9-15]. These studies revealed that reaction medium was one of important factor to control the structural and the morphological properties of ceramics. The solid-state reaction method has been commonly used in industry to produce essential materials for industrial applications because of its simplicity and low cost [16]. This method is adopted in the production of Barium titanite and the process can be summarized:
A. BaCO3 and TiO2 powders are mixed in a high energy ball mill to produce small particle size powders4. The powder is mixed with solvents such acetone, methanol or water to form a homogenized slurry [14-18]. Theoretically, there is no reaction between the liquids and the reagents, but there is no experimental evidence is available.
B. BaCO3 and TiO2 powders do not react at room temperature so the mixture is calcined about 1000 °C in order for the reaction that have been investigating in many articles [16,17].
C. After calcination, powder is pressed into pellets and pellets sintered to investigate electrical properties [4].
The rate of solid-state reaction and phases in powders is very sensitive to reactants, temperature, and synthesis conditions [16-22]. However, homogenization medium effect on electrical properties of BaTiO3 ceramics by solid-state reaction method has not been investigated. In this study, BaTiO3 powders were homogenized in three different media; distilled water, methanol and acetone. Influence of homogenization medium on structure, morphology and dielectric properties of ceramics was investigated.
Materials and Methods
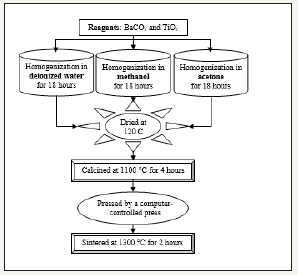
BaTiO3 were prepared by solid-state process that reagents homogenized in distilled water, methanol and acetone. Schematic diagram of solid-state process is given in Figure 1.
Figure 1:Schematic diagram of solid-state process.
Materials
Analytical grade (purity>99.5%) BaCO3 and TiO2 (Alfa-Aesar, United Kingdom) powders. Distilled water (Merck-Millipore Direct-Q, Deutschland), methanol and acetone (Merck Darmstadt, Deutschland).
Homogenization and calcination
BaCO3 and TiO2 powders were mixed inside polyethylene sample vials with 5mm diameter zirconia balls in distilled water, methanol and acetone. The vials were shaken simultaneously by Compact Mixer Shaker (Edmund Bühler, Deutschland) at 200rpm with motion orbital for 18 hours to homogenize and to de-agglomerate the powders. After homogenization, all sludge (muddy) mixtures were dried at the same time. The powders were calcined at 1100 °C for 4 hours in alumina crucibles together.
Structural analysis
The structure of the powders was characterized by X-ray diffraction (XRD) (Rigaku-Smart Lab, Japan) with diffractometer in the range 10° ≤ 2θ ≤ 90° with CuKα radiation (40kV, 30mA, step:0.02).
Sinterization
The calcined powders were pressed by a computer-controlled press machine into disc-shaped pellets, 10mm in diameter and 2mm thick. The pellets were sintered at 1300 °C for 2 hours at the same time.
Morphological analysis
Morphology of the sintered pellets were analyzed using Scanning Electron Microscopy (SEM) (JEOL-5500, Japan).
Dielectric measurement
The complex impedance measurements of samples were carried out using high-performance dielectric spectrometer (NOVOCONTROL-BDS41, Deutschland) with ZGS Active Sample Cell at oscillation amplitude of 1V in a wide frequency range of 100mHz- 20MHz at room temperature. Win DETA Novo control software was used for impedance data analysis.
Results and Discussion
Microstructural and morphological analysis
Table 1:Values of ε∞ , n, viscosity of liquids of homogenization medium and density of calcined powders.
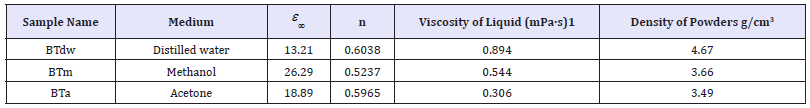
Microstructural and morphological analysis of the pellets discussed in detail using XRD patterns and SEM images of the samples in previous research [22]. According to the XRD data results, the predominant phase of the ceramics is BaTiO3. But according to SEM images, the morphology of the materials strongly depends on the homogenization medium. First sample, prepared in distilled water (BTdw), have a continuous structure with extremely large grains with no cavities or pores between its grain walls on the surface. Second sample, prepared in methanol (BTm), have a continuous and big grain size on the structure with some cavities, cracks, and holes between its grain boundaries. Third sample, prepared in acetone (BTa), have heterogeneous and discontinuous structure with different grain sizes. Densification of pellets is correlated to the average particle size of powders that uniform powder with small average particle size improved densification of ceramics prepared by solid-state reaction with good dielectric properties [11-23]. Densities of each sample after calcinations were measured by using the Archimedes method. The calcined powders densities were 4.67, 3.66, and 3.49g/cm3 for BTdw, BTm, and BTa respectively. It seems that density of the powders is correlated to the viscosity of liquid given in Table 1. It may be explained that the powders in shaker were exposed to different friction because of the viscosity of liquids. De-agglomeration of the powders depends on friction because powders subjected to different collision energy from the balls in different mediums. According to XRD, SEM and density results, using distilled water as homogenization medium decreases the particle size of the powder and result in more uniformed powders, which leads to dense ceramics.
Impedance spectroscopy analysis
Different electrical response of materials like ionic, electronic, and interfacial can be analyzed with complex permittivity (ε ^ * = ε ^ ' − jε ") and complex electric modulus (M ^ * = M ^ '+jM " = 1 / ε ^ *) , where (ε ^ ',M ') and (ε ",M ") are real and imaginary parts of permittivity and electric modulus respectively; j = √ (−1) and C0=vacuum capacitance. There are four mechanisms which may lead to polarization in ceramics:
A. Interfacial and space charge,
B. Dipolar polarization,
C. Ionic, and
D. Electronic polarizations.
All types of polarization mechanism could contribute to dielectric constant at low frequency region. Some types of polarizations may have less contribution in permittivity in high frequency region. Decreasing in ε ^' at lower frequency region is due to interfacial polarization and hopping mechanism in the material. This effect can be explained with Maxwell-Wagner-Sillars (MWS) polarization (interfacial polarization). The interfacial polarization happens at the boundaries between the different microstructures (i.e. crystalline and non-crystalline regions, grains and grain-boundaries) in samples because of space charges, which contribute high dielectric constant at lower frequencies. In hopping mechanism at lower frequency region, charge carriers hope from one side to neighboring side under an electric field. Ionic and electronic polarizations are frequency independent in high frequency region because space charges have not enough time to orient themselves in the direction of the signals [24].
Figure 2 shows real (ε ^') and imaginary (ε ") parts of the sintered pellets permittivity. ε ^' and ε "of ceramics decrease with increasing frequency. ε ^' and ε " values of BTm sample are higher than ε ^' and ε " values of BTdw and BTa. ε ^' and ε " of samples have a maximum value at the lowest frequency and decrease very fast with increasing frequency up to 10Hz. Decreases of ε ^' and ε "of BTm sample are the fastest one. ε ^' decreases slowly in intermediate frequency region between 10Hz-1kHz. Above 1kHz, ε ^' and ε " are almost frequency-independent. Due to accumulation of electric charges at the interfaces of the samples homogenized in methanol, the dielectric constant of the ceramic is considerably elevated. ε " for ceramics has similar behavior like real part of permittivity. ε " results from imperfections in the ceramic such as grain boundaries, porosity, spots etc. and decreases with increasing grain boundary resistivity [3]. Depending on different structure and microstructure of samples seen at SEM images and XRD patterns given in Figure 2, samples have different conduction and relaxation mechanism. Curie-von Schwedler (CS) function gives the relation of ε ^'(ω) = ε _ ∞ + Bω ^(n-1) with frequency. Where ε _ ∞ is high-frequency limit of permittivity; B is a constant; ω = 2πf is angular frequency; and n is relaxation parameters ( 0 ≤ n ≤ 1 ; n=1 is Debye relaxation) [24]. The values of ε _ ∞ , n and density of powders for samples have been obtained from CS function shown in Table 1. The calculated value of n< 1 indicates that samples have non-Debye type relaxation.
Figure 2:Frequency dependence of permittivity of samples a) Real part b) Imaginary part.
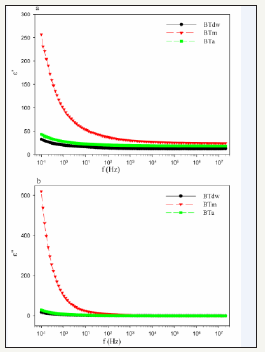
Figure 3:Frequency dependence of electric modulus of samples a) Real part b) Imaginary part
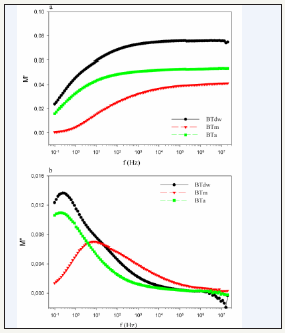
The complex modulus with frequency of sintered pellets is shown in Figure 3. The real part of electric modulus (M ^') for all the samples has an increasing trend with increasing frequency up to 10Hz. It is clear that increasing (M ^') is slowly in frequency region between 10Hz -1kHz and tends to reach a constant value above 1kHz. This behavior shows that materials have a continues dispersion with increasing frequency because of possible contribution to the conduction in the materials due to short range mobility of charge carriers under the electric field. That could be explained the electrical properties of ceramics come up due to intragrain sizes and shapes [4]. The peak of M " for BTdw and BTa is the lowest frequency besides M " peak for BTm shifts towards higher frequencies. M " plots show a peak at low frequencies. Charge carriers can move a long distance in the materials below the peak frequency while charge carriers mobile short distance in materials because of confined potential wells. The wide peak shape could be explained that the materials have a relaxation time with the different mean time constant (MWS type relaxation) [18]. The charge carriers accumulate at interfaces or charge carriers hope from one side to the neighboring side under an electric field [24].
Figure 4:Frequency dependence of normalized electric modulus vs f/fmax of samples.
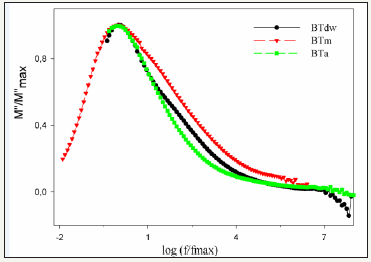
The relaxation process in material could be also analyzed with normalized electric modulus formalism by using relationship

Conclusion
BaTiO3 ceramics formed by solid-state reaction of BaCO3 and TiO2 were homogenized in distilled water, methanol, and acetone medium to investigate effect of homogenization medium on dielectric properties. XRD pattern and SEM images showed that predominant phase of samples is BaTiO3, but crystallinity of samples changes significantly with homogenization medium. Depending on the homogenization medium, sintered pellets have continuous structure without any pores for BTdw, continuous structure with some pores for BTm, and discontinuous structure for BTa. The complex permittivity and complex electric modulus analysis of ceramics indicated that the materials have the relaxation time with different mean time constant. Minimum values of ε ", represents the dielectric loss in ceramic, found ceramic that its reagents homogenized in distilled water, which is very important for many electronic applications. This effect may be explained with the temperature used in this process which is able to remove the water totally due to its small molecules structure. The result presented here expose the fact that different homogenization medium changes microstructure of BaTiO3 ceramics which is a major effect on the electrical properties. Complex permittivity and resistivity measurements showed that using water has a positive effect on electrical properties of barium titanate ceramics; due to the powders have obtained pure and uniform powders with small average particle size. The powders were obtained highly crystalline, which is important for achieving the best dielectric properties of the ceramics. In the other words the packing (green) density of the ceramic homogenized in water were increased because the powders were obtained pure, uniform and highly crystalline, which lead to achieving the best dielectric ceramics with the smallest dielectric loss.
Acknowledgement
The authors thank to Scientific Research Projects Coordination Unit for project funding by grant no: 196, Mustafa Kemal University, Hatay.
References
- Lin Y, Zhou Z, Sodano HA (2013) Barium titanate and barium strontium titanate coated carbon fibers for multifunctional structural capacitors. J Compos Mater 47(12): 1527-1533.
- Yuasa M, Nagano T, Tachibana N, Kida T, Shimanoe K (2013) Catalytic combustion-type hydrogen sensor using BaTiO3-ased PTC thermistor. J Am Ceram Soc 96(6): 1789-1794.
- Yang SL, Wu JM (2011) Effects of Nb2O5 in (Ba,Bi,Nb) added TiO2 ceramic varistors. J Mater Res 10(2): 345-352.
- Sharma S, Shamim K, Ranjan A, Rai R, Kumari P, et al. (2015) Impedance and modulus spectroscopy characterization of lead free barium titanate ferroelectric ceramics. Ceram Int 41(6): 7713-7722.
- Roy AK, Prasad K, Prasad A (2013) Piezoelectric, impedance, electric modulus and AC conductivity studies on (Bi0.5Na0.5)0.95Ba0.05TiO3 ceramic. Process Appl Ceram 7(2): 81-91.
- Garbarz GB (2014) Impedance and modulus spectroscopy of a novel ferroelectric ceramics based on Barium Titanate. Ferroelectrics 463(1): 90-98.
- Ganguly P, Jha A (2010) Impedance spectroscopy analysis of Ba5NdTi3Nb7O30 ferroelectric ceramic. Phys B Condens Matter 405(15): 3154-3158.
- Manzoor U, Kim D (2007) Synthesis of nano-sized barium titanite powder by solid-state reaction between barium carbonate and Titani. J Mater Sci Technol 23: 655-658.
- Yoon S, Baik S, Kim MG, Shin N, Kim I (2007) Synthesis of Tetragonal Barium Titanate nanoparticles via alkoxide? Hydroxide sol-precipitation: Effect of water addition. J Am Ceram Soc 90(1): 311-314.
- Qi JQ, Peng T, Hu YM, Sun L, Wang Y, et al. (2011) Direct synthesis of ultrafine tetragonal BaTiO3 nanoparticles at room temperature. Nanoscale Res Lett 6(1): 466.
- Marković S, Miljković M, Jovalekić Č, Mentus S, Uskoković D (2009) Densification, microstructure, and electrical properties of BaTiO3 (BT) ceramics prepared from ultrasonically de-agglomerated BT powders. Mater Manuf Process 24(10-11): 1114-1123.
- Cho WS, Hamada E (1988) Synthesis of ultrafine BaTiO ${3}$ particles from polymeric precursor: Their structure and surface property. J Alloys Compd 266(1-2): 118-122.
- Kwon SG, Choi K, Kim BI (2006) Solvothermal synthesis of nano-sized tetragonal barium titanate powders. Mater Lett 60 (7): 979-982.
- Moon SM, Lee C, Cho NH (2006) Structural features of nanoscale BaTiO3 powders prepared by hydro-thermal synthesis. J Electro ceramics 17(2- 4): 841-845.
- Stawski TM, Veldhuis SA, Göbel OF, Ten Elshof JE, Blank DHA (2010) Effects of reaction medium on the phase synthesis and particle size evolution of BaTiO3. J Am Ceram Soc 93(10): 3443-3448.
- Manzoor U, Kim DK (2007) Synthesis of nano-sized barium titanate powder by solid-state reaction between barium carbonate and titania. J Mater Sci Technol 23(5): 655-658.
- Badapanda T, Senthil V, Rana DK, Panigrahi S, Anwar S (2012) Relaxor ferroelectric behavior of ‘A’ site deficient Bismuth doped Barium Titanite ceramic. J Electro ceramics 29(2): 117-124.
- Shukla A, Choudhary RNP, Thakur AK (2009) Thermal, structural and complex impedance analysis of Mn4+ modified BaTiO3 electroceramic. J Phys Chem Solids 70(11): 1401-1407.
- Vijatović P, Bobić JD, Uršič H, Banys J, Stojanović BD (2013) The electrical properties of chemically obtained barium titanate improved by attrition milling. J Sol-Gel Sci Technol 67(2): 267-272.
- Felgner KHH, Müller T, Langhammer HTT, HPP Abicht (2004) On the formation of BaTiO3 from BaCO3 and TiO2 by microwave and conventional heating. Mater Lett 58(12-13): 1943-1947
- Zhong W, Zhang P, Wang Y, Ren T (1994) Size effect on the dielectric properties of BaTiO3. Ferroelectrics 160(1): 55-59.
- Akbas HZ, Aydin Z, Guder F, Turgut S (2017) Accelerated formation of BaTiO3 ceramics with mechanochemical processing in different liquids. J Alloys Compd 699: 87-91.
- Ring TA (1996) Fundamentals of ceramic powder processing and synthesis.
- Khan MH, Pal S, Bose E (2015) Frequency-dependent dielectric permittivity and electric modulus studies and an empirical scaling in (100-x) BaTiO3/(x)La07Ca03MnO3 composites. Appl Phys A 118(3): 907- 912.
- Verma K, Sharma S (2012) Influence of addition of copper cadmium ferrite on the dielectric and electrical behavior of BaSrTiO3 ceramics. Ceram Int 38(7): 5957-5966.
- Lide D, Taylor Fr, Boca RFL (2007) CRC handbook of chemistry and physics.
© 2018 Hatice Zehra Dolek. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






