- Submissions

Full Text
COJ Reviews & Research
The Neural Metarteriole-Angiocentric Glioma
Anubha Bajaj*
Department of Histopathologist in A B Diagnostics, India
*Corresponding author: Anubha Bajaj, Department of Histopathologist in A B Diagnostics, India
Submission: July 01, 2022; Published: July 25, 2022

ISSN 2639-0590Volum4 Issue1
Introduction
Angiocentric glioma is an exceptional, epileptogenic, diffuse, paediatric-type, low grade glioma which commonly emerges within superficial, supra-tentorial region of the brain. Confined to the cerebral cortex, the central nervous system neoplasm exhibits feature identical to an infiltrating astrocytoma and ependymoma. World Health Organization classifies the indolent angiocentric glioma as a grade I, specifically as a “paediatric type, diffuse, lowgrade glioma. Currently, high grade angiocentric gliomas are devoid of distinctive diagnostic criterion. Angiocentric glioma is frequently discerned within cortical and sub-cortical areas of supra-tentorial region. The neoplasm depicts a propensity to extend horizontally within a plane subjacent to pia mater and within deep seated areas along diverse vascular articulations.
Disease Characteristics
Angiocentric glioma is usually situated within the brainstem, pons, midbrain, pons and medulla or pons and cerebellum. The well delineated, cortical, or subcortical angiocentric glioma is confined to the junction between grey matter and white matter where it characteristically expands incriminated gyri. Angiocentric glioma occurring within the brainstem appears in younger subjects, in contrast to supra-tentorial neoplasms and is usually un-associated with seizures or epilepsy [1,2]. Although the neoplasm predominantly appears within cortical and subcortical zones, the brainstem, subcortical area, diffuse lesions, and the thalamus may be incriminated (Figure 1). Cystoid component is delineated in beyond >half the neoplasms [1,2]. Generally, children and young adults are incriminated, and the neoplasm is discerned below <20 years. No age of tumour emergence is exempt although median age of tumour discernment is 13 years and mean age is at 17 years. A male predominance is observed with a male to female proportion of around 2.5:1 or the neoplasm may exhibit an equivalent gender predominance [1,2]. Of obscure aetiology, angiocentric glioma may simulate an ependymoma or exhibit a distinct ependymoma-like component. However, concordance of angiocentric glioma with ependymoma remains ambiguous [1,2]. It is posited that epileptogenesis concurrent with angiocentric glioma may be engendered due to frequent occurrence of the neoplasms within cerebral cortex [1,2].
Figure 1: Angiocentric glioma depicting perivascular aggregates of monomorphic, bipolar, spindle-shaped cells within the cerebral cortex.
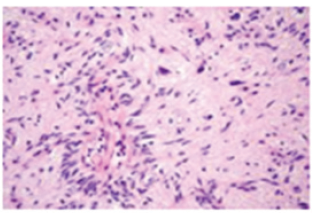
Clinical Elucidation
Characteristically, the superficial angiocentric glioma demonstrates seizures, variants of epilepsy or intractable partial epilepsy which is unresponsive to pertinent medical management (Figure 2). Angiocentric gliomas confined to the brain stem demonstrate facial hemiparesis, recurrent pneumonia, diplopia, irregular gait, and headache with vomiting although history of seizures or epilepsy is usually absent. Neoplasms emerging within the brainstem commonly manifest clinical symptoms necessitating imaging of the brain such as cranial nerve palsy and intracranial hypertension [3,4]. Angiocentric glioma of significant duration is associated with increased incidence of tumour-associated seizures or epilepsy-induced brain atrophy and stalk-like tumour extension [3,4]. Majority of incriminated individuals display a prolonged history of intractable seizures or epilepsy prior to cogent surgical resection. Characteristic features on magnetic resonance imaging as intra-tumoral elevated signal intensity upon T1 weighted imaging, stalk-like sign and atrophy of brain parenchyma circumscribing the neoplasm may appear on account of indolent angiocentric glioma associated with intractable seizures or epilepsy of significant duration or coexistent Focal Cortical Dysplasia [FCD] and gliosis [3,4]. Focal Cortical Dysplasia [FCD] may initiate intractable seizures or epilepsy of significant duration. Besides, extensive seizures or prolonged history of epilepsy may contribute to regional atrophy and degeneration of brain parenchyma circumscribing the neoplasm [3,4].
Figure 2: Angiocentric glioma delineating accumulated monomorphic, bipolar, spindle-shaped cells circumscribing vascular articulations.
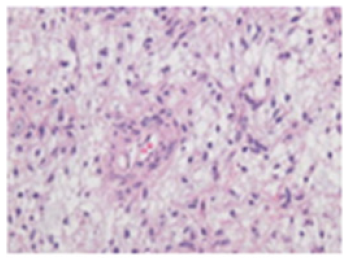
Histological Elucidation
Upon gross examination, a firm, poorly defined neoplasm is observed [5,6]. Upon microscopy, the infiltrative neoplasm is composed of monomorphic, bipolar, spindle-shaped cells configured in an angio-centric pattern while circumscribing cortical vascular articulations. Foci of ependymoma-like pseudorosettes with palisading subjacent to the pia-mater, accumulated tumour cells and miniature schwannoma-like nodules can be exemplified [5,6]. Tumefaction is composed of monomorphic population of elongated, spindle-shaped, bipolar cells which appear disseminated within the perivascular region, simulating perivascular pseudo-rosettes (Figure 3). Although tumour cells expand towards circumscribing cerebral parenchyma, the cells depict an intense predilection for perivascular dissemination [5,6]. Distribution of tumour cells within subjacent pia-mater or along superficial cerebral cortex is predominant [5,6]. Generally, mitotic figures, focal vascular proliferation and tumour induced necrosis is absent. Lesions exhibiting significant mitosis are associated with enhanced possible tumour reoccurrence [5,6]. Tumour cells are focally immune reactive to Epithelial Membrane Antigen [EMA] within surface zones or may depict perinuclear or cytoplasmic, dot-like micro lumina. Variable immune reactivity to Glial Fibrillary Acidic Protein [GFAP] is observed. Ki-67 proliferation index is usually beneath <5% (Figure 4). Tumour cells do not react to Isocitrate Dehydrogenase [IDH] [5,6]. Tumour cells are immune non-reactive to synaptophysin, chromogranin-A and neuronal nuclear antigen [5,6].
Figure 3: Angiocentric glioma demonstrating perivascular clusters of monomorphic, bipolar, spindleshaped cells scattered amidst the cerebral cortex.
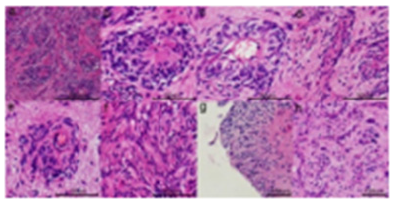
Figure 4: Angiocentric glioma exhibiting fascicles of monomorphic, bipolar, spindle-shaped cells with a predilection for vascular articulations.
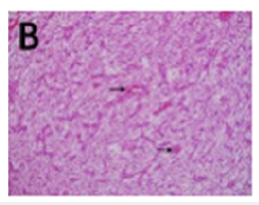
Differential Diagnosis
Angiocentric glioma requires a distinction from neoplasms of central nervous system such as astroblastoma which is a welldefined neoplasm with a discrete, pushing, well circumscribed tumour perimeter. Tumefaction is composed of epithelioid cells. Focal vascular sclerosis can be delineated [7,8]. Occasionally, lesions of advanced grade appear infiltrative (Figure 5). Perivascular pseudo-rosettes can be discerned, akin to ependymoma although cellular processes can be thick and extend from cell body to vascular adventitia. Foci of vascular hyalinization can be observed along with minimal fibrillary background [7,8]. infiltrating astrocytoma is a neoplasm which depicts an intra-fascicular pattern of tumour evolution along white matter tracts and lacks pseudo-rosettes. Palisading of tumour cells subjacent to pia-mater is absent [7,8]. Tumour evolution is diffuse and infiltrative. Tumour cells expand along pre-existing cellular structures within grey matter and white matter tracts with configuration of secondary structures of Scherer comprised of peri-neuronal satellitosis, perivascular satellitosis with sub-ventricular and sub-pial accumulation of cells [7,8]. Ependymoma is a neoplasm with characteristic discrete, sharply circumscribed tumour perimeter. The cellular neoplasm may be infiltrative and is comprised of monomorphic spherical to elliptical cells imbued with speckled chromatin. Perivascular pseudorosettes, true ependymal rosettes, well defined cellular lumen, and foci of fibrillary areas may be discerned.
Figure 5: Angiocentric glioma exemplifying bundles of monomorphic, bipolar, spindle-shaped cells enveloping vascular channels.
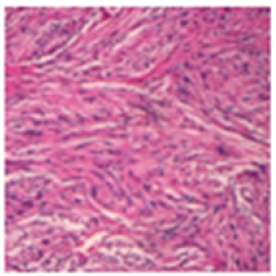
Tumefaction arising within posterior fossa can depict gametocyte-like cells and hyper-cellular nodules. Foci of nonpalisading tumour necrosis, cystic or myxoid degeneration, calcification, degenerative cellular or nuclear atypia, neuronal differentiation and infrequent, metaplastic components can be delineated. Ependymoma can display diverse morphological subtypes as papillary, clear cell and tanycytic, categories which are devoid of pertinent clinical or pathological significance. Tumefaction manifests as an enhancing lesion [9,10]. ganglioglioma or gangliocytoma emerges as a well-defined, “bubbly”, compact tumefaction with a variable, lobular architecture confined to cortical or subcortical region with minimal circumscribing oedema. Neoplastic glial component is admixed with proliferative, neoplastic ganglion cells (Figure 6). The astrocytic element simulates fibrillary astrocytoma or pilocytic astrocytoma. Mitosis is exceptional and tumour necrosis is usually absent. Generally, the neoplasm depicts a perivascular cuff of mature, small lymphocytes. Besides, Rosenthal fibres and eosinophilic granular bodies can be discerned.
Figure 6: Angiocentric glioma displaying perivascular nests of monomorphic, bipolar, spindle-shaped cells spread within the cerebral cortex.
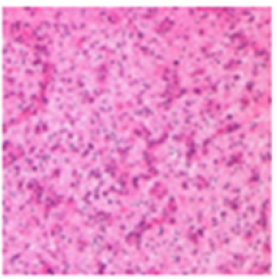
Tumefaction displays micro-cysts, a prominent network of reticulin, focal calcification, vascular proliferation with configuration of glomeruli structures and desmoplasia, especially in neoplasms emerging within the subarachnoid space. Ki-67 proliferative index is minimal at up to 2.7%. The neoplasm is associated with image enhancement upon magnetic resonance imaging. Tumour perimeter exhibits an elevated signal intensity upon Fluid-Attenuated Inversion Recovery [FLAIR] [9,10]. Besides, angiocentric glioma requires a segregation from diffuse gliomas as astrocytoma, oligodendroglioma, focal cortical dysplasia, cortical tuber or variant tuberous sclerosis, perivascular spaces along with multinodular and vacuolated neuronal tumours comprised of subcortical aggregates of cyst-like lesions [9,10].
Investigative Assay
Appropriate assessment of cogent clinical manifestations, history of seizures or epileptic fits, adopted therapeutic strategies, tumour reoccurrence following comprehensive surgical extermination, duration between preliminary surgical intervention and tumour reoccurrence, proportionate survival and period of tumour monitoring is necessitated [9,10]. Additionally, signal intensities of non-cystoid and cystoid components of cortical neoplasms can be compared with T1 weighted imaging, T2 weighted imaging and Fluid-Attenuated Inversion Recovery [FLAIR]. Pattern of contrast enhancement and diffusion restriction is discerned along with assessment of mean apparent diffusion coefficient. Massive oedema circumscribing the neoplasm where magnitude of oedema is ≥ to tumour dimension and atrophy of brain parenchyma abutting the neoplasm can be evaluated [11,12]. Besides, evaluation of tumour magnitude, laterality, site of neoplasm, tumour perimeter and incrimination of cortex or subcortex is required. Upon computerized tomography, the neoplasm typically demonstrates an expansible, non-enhancing tumefaction confined to cerebral cortex. Magnetic Resonance Imaging [MRI] denominates distinctive features of angiocentric glioma [11,12].
Upon T1 weighted imaging, a decimated signal intensity is observed whereas tumour perimeter depicts an enhanced signal intensity (Figure 7). T2 weighted imaging and fluid-attenuated inversion recovery [FLAIR] depicts stalk-like lesions of elevated signal intensity which extend to the ventricles along vascular configurations. Cystic tumour foci may be discerned [11,12]. Stalklike sign which is apparent upon T2 weighted imaging or FLAIR sequencing is denominated as a hyper-intense signal which tapers toward lateral ventricles [11,12]. Upon T2 weighted imaging, the cystoid component denominates a non-enhancing tumour segment with elevated signal intensity, akin to cerebrospinal fluid whereas signal intensity is decimated upon T1 weighted imaging [11,12]. Generally, a well demarcated, solid, hyper-intense, non-enhancing tumefaction appears confined to the cerebral cortex. Besides, stalklike extension into adjacent ventricle is a pathognomonic feature [11,12]. Intra-tumoral areas of enhanced signal intensity upon T1 weighted imaging, non- enhancement, superficial tumour location, stalk-like sign upon T2 weighted imaging and FLAIR, atrophy of brain parenchyma abutting the tumefaction and adjacent Focal Cortical Dysplasia [FCD] are characteristic manifestations [13,14]. Nevertheless, stalk-like sign may be infrequent and contrast enhancement may occur in exceeding > one-fourth of neoplasms. Intra-tumoral elevated signal intensity upon T1 weighted imaging is common in subjects with regional cortical atrophy and stalk-like signs [13,14].
Figure 7: Angiocentric glioma enunciating aggregates of monomorphic, bipolar, spindle-shaped cells with a propensity for perivascular dissemination.

Occurrence or absence of contrast enhancement of tumour manifestations with T1 weighted imaging can be assayed. Elevated signal intensity upon post contrast T1 weighted imaging may indicate absence of contrast enhancement (Figure 8). Tumour enhancement upon administration of gadolinium contrast is absent wherein angiocentric gliomas emerging within the brainstem appear devoid of contrast administration [13,14]. Also, intra-tumoral, preenhanced elevated signal intensity upon T1 weighted imaging can be discerned [13,14]. Additionally, assessment of duration of commencement of seizures or epilepsy, occurrence, or absence of intra-tumoral elevated signal intensity upon T1 weighted imaging, stalk-like sign and atrophy of brain parenchyma circumscribing the neoplasm is beneficial [13,14]. Duration of seizures or epilepsy is significantly increased in subjects demonstrating intra-tumoral elevated signal intensity upon T1 weighted imaging, stalk-like signs, and regional atrophy of brain parenchyma [13,14]. Enhanced signal intensity occurring with T1 weighted imaging of angiocentric glioma is indicative of various chronic processes [13,14]. Emergence of stalk-like sign connotes diverse pathological incursions such as tumour infiltration along extending vascular configurations from adjoining brain surface towards the ventricles, peritumoral gliosis initiated by neoplasms of significant duration or seizureinduced oxidative stress and coexistent Focal Cortical Dysplasia [FCD] [13,14]. Assessment of cogent features on spectroscopy and tumour perfusion upon magnetic resonance imaging is necessitated [13,14]. Dynamic susceptibility contrast perfusion MRI exemplifies increase in cerebral blood flow and volume [13,14]. Upon Magnetic Resonance [MR] spectroscopy, decimated values of N-acetyl aspartate, elevated creatine and choline and peaking of lactate values is observed [13,14].
Figure 8: Angiocentric glioma depicting hyper-intense lesion with elevated signal intensity confined subjacent to the pia-mater.
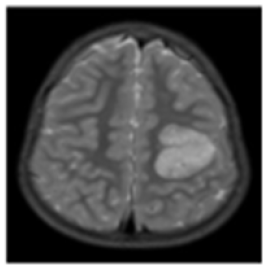
Therapeutic Options
Comprehensive surgical eradication of the neoplasm is an optimal and commonly employed treatment strategy. Surgical extermination demonstrates amelioration of seizures or various forms of epilepsy [14,15]. Employment of radiotherapy or chemotherapy remains debatable. Therapeutic outcomes are favourable and tumour reoccurrence is exceptional. Besides, tumour reoccurrence is decimated following complete surgical resection [14,15].
References
- Kurokawa R, Baba A, Pinarbasi E, Mariko K, Yoshiaki O, et al. (2022) Neuroimaging features of angiocentric glioma: A case series and systematic review. J Neuroimaging 32(3): 389-399.
- Ampie L, Choy W, Joseph DD, Jonathan BL, Christopher, et al. (2016) Clinical attributes and surgical outcomes of angiocentric gliomas. J Clin Neurosci 28: 117-122.
- Harmsen H, Mobley BC (2019) Angiocentric glioma mimicking encephalomalacia. Radiol Case Rep 14: 700-133.
- Han G, Zhang J, Yue M, Gui Q, Yin S (2020) Clinical characteristics, treatment, and prognosis of angiocentric glioma. Oncol Lett 20(2): 1641-1648.
- Halloran PJ, Amoo M, Dablouk M, Alan B, Stephen M (2020) Angiocentric glioma: drop metastases to the spinal cord. World Neurosurg 136: 110-116.
- Taschner CA, Sankowski R, Urbach H, Storz C (2019) Freiburg neuropathology case conference: hyper salivatory seizures in a 6-year-old child. Clin Neuroradiol 29: 581-286.
- Aronco LD, Rouleau C, Gayden T, Crevier L, Decarie JC, et al. (2017) Brainstem angiocentric gliomas with MYB-QKI rearrangements. Acta Neuropathol 134(4): 667-669.
- Weaver KJ, Crawford LM, Bennett J, Marie LRZ, Pincus D, et al. (2017) Brainstem angiocentric glioma: report of 2 cases. J Neurosurg Pediatr 20(4): 347-351.
- Gonzalez Quarante LH, Fernández Carballal C, Vijay A, Oscar LS, Emma SV, et al. (2017) Angiocentric glioma in an elderly patient: case report and review of the literature. World Neurosurg 97: 755.e5-755.e10.
- McCracken JA, Gonzales MF, Phal PM, Drummond KJ (2016) Angiocentric glioma transformed into anaplastic ependymoma: review of the evidence for malignant potential. J Clin Neurosci 34: 47-52.
- Adamek D, Siwek GP, Chrobak AA, Sucharska IH, Swiatkowski S, et al. (2016) Angiocentric glioma from a perspective of A-B-C classification of epilepsy associated tumours. Folia Neuropathol 54(1): 40-49.
- Cheng S, Lü Y (2015) Cystoid angiocentric glioma: a case report and literature review. J Radiol Case Rep 9: 1-9.
- Ersen A, Canda MS, Men S, Yucesoy K, Kalemci O, et al. (2017) Angiocentric glioma: the infiltrative glioma with ependymal differentiation. Turk Patoloji Derg 33(3): 251-255.
- Ni HC, Chen SY, Chen L, Lu DH, Fu YJ, et al. (2015) Angiocentric glioma: a report of nine new cases, including four with atypical histological features. Neuropathol Appl Neurobiol 41(3): 333-346.
- Almubarak AO, Alahmari A, Hindi HA, Alshail E (2020) Angiocentric glioma of brainstem. Neurosciences (Riyadh) 25(5): 416-420.
© 2022 Anubha Bajaj. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






