- Submissions

Full Text
COJ Reviews & Research
Microbial Mediated Soil Carbon Sequestration and Mitigation of Green House Gases
Pooja Arora* and Smita Chaudhry
Institute of Environmental Studies, India
*Corresponding author:Pooja Arora, Institute of Environmental Studies, Kurukshetra University, Kurukshetra, Haryana, India
Submission: July 06, 2021; Published: August 19, 2021

ISSN 2639-0590Volum3 Issue3
Abstract
Anthropogenic development activities have resulted in rapid global climate change. The effects of increasing surface temperatures are evident from unprecedented loss in plant and animal species. However, microbial life has not been well discussed in context of climate change. The present review has been done to understand the basic processes that soil microbial communities carry out in context of climate change such as microbial mediated soil carbon sequestration and mitigation of greenhouse gases. Soil microbes play a crucial role in decomposition of organic matter and soil respiration processes that are fundamental in sequestering carbon in the soil and CO2 emissions from the soil. The emissions of other important greenhouse gases such as methane and nitrous oxide are also mitigated by microbial actions. The mitigation of methane is brought about by its oxidation by methanotrophic bacteria while that of nitrous oxide is achieved by manipulating soil microbiome in situ along with manipulation in biotic and abiotic factors in soil. Hence, an understanding of the role of soil microbes as both contributors to and reactive components of climate change is much needed to clarify their roles whether they can be used to mitigate the emissions of GHGs or accelerators of climate disaster at the macroscopic level and even at the global scale.
Keywords: Soil microbes; Carbon sequestration; Climate change; Mitigation; Greenhouse gases; Carbon dioxide; Methane; Nitrous oxide
Introduction
Earth’s environment has been undergoing changes due to increasing human population
and its activities. The most significant change is the increase in concentration of carbon
dioxide and other greenhouse gases in the troposphere resulting in increase in average
surface temperature of Earth. The change in climate is the result of continuous but nonlinear
interaction among components of the climate system at varying time scales (minutes
to billions of years). But the “enhanced greenhouse effect” and resulting rapid global climate
change is posing great threat to the well-being of humankind and other life forms on the earth
as evident from unprecedented loss in plant and animal species. By contrast, microbial life has
not been well discussed in context of climate change.
Although invisible to the naked eye, the abundance and diversity of microorganisms is
very crucial in determining the resilience of many organisms and ecosystems and thereby
their ability to respond to changing climate scenarios. A typical soil sample may contain more
than 106 individual species-level Operational Taxonomic Units of Bacteria, Archaea, and
Fungi Fierer et al. [1]. Estimation of soil microbial communities and biomass is of fundamental
importance to study a wide range of soil processes such as nutrient cycling, organic matter
decomposition, soil quality, soil respiration and mineralization. Hence, microbes can be
viewed as the engines that drive these processes.
Climate Change mitigation necessitates the management of terrestrial carbon (C) by
creating new C sinks and also by preserving and enhancing the existing ones. Carbon capture
in living biomass, in soil (roots and microbes) and recalcitrant organic and inorganic carbon all in terms of carbon sequestration is one such option. Even the
seasonal variations of soil microbial biomass as a potential stock of
carbon reflect the degree of immobilization and mineralization of
soil carbon Chaudhry [2]. Microbial diversity in different ecosystem
has many contributions in controlling the climate change and
combating its negative impacts owing to their amazingly versatile
metabolism and ability to flourish in broad environmental
conditions.
Microbial Mediated Soil Carbon Sequestration
Soil micro-organisms have a crucial role in cycling of the nutrients, promoting and accelerating the decomposition of the organic matter, the release of nutrients contained therein and précising the processes that control the flow of these nutrients to plants and hydrological & gaseous losses to surrounding environments. The dominating factor in the terrestrial carbon cycle is the balance between two processes of photosynthesis and respiration. Autotrophic organisms, especially photosynthesizing plants, and also photo and chemoautotrophic microorganisms fix atmospheric carbon dioxide (CO2) into organic material (Figure 1); [3] which is then returned back to atmosphere via autotrophic and heterotrophic respiration and decomposition pathways. Decomposition is carried out by ‘organic carbon-consuming’ heterotrophic microorganisms that feed on carbon of either plant, animal or microbial origin as a substrate. Some amount of this carbon is retained in their biomass while the rest is released as metabolites or as CO2 back to the atmosphere. In this way, soil microorganisms directly regulate the amount of organic carbon stored in soil and released back to the atmosphere. They also indirectly influence carbon storage in plants and soils by providing macronutrients (N & P) to regulate productivity Singh et al. [4], Bardgett [5]. Further, in many ecosystems, mycorrhizal fungi are responsible for substantial amounts of nitrogen and phosphorus acquisition by plants Fellbaum et al. [6].
Figure 1: Microbial mediated terrestrial carbon cycle Gouglias C et al. [3].
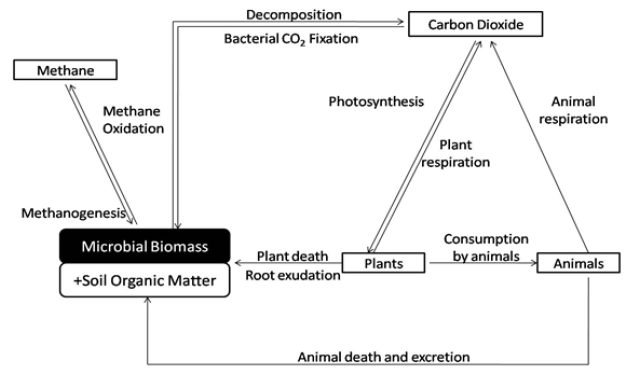
Therefore, many studies suggest that soil microorganisms have a key role in controlling formation, decomposition and accumulation of Soil Organic Matter Mellado-Vazquez et al. [7]; Liang et al. [8]; Cotrufo et al. [9]; Lange et al. [10]; Balser [11]; Gleixner et al. [12]. In vice versa situation, plant derived C resources largely influence the soil microbial activity apart from other factors such as soil temperature, soil humidity or pH Voroney [13]. Also, the impact of different photosynthetic pathways on the microbial community may be triggered by confounding effects of other environmental parameters Chen et al. [14].
Decomposition of Soil Organic Matter
Soil Organic Matter (SOM) consists of fresh to progressively
decomposing plant, microbial and faunal-derived debris and
exudates. The microbial biomass, responsible for primary
decomposition of these materials also forms a part of Soil Organic
Matter. The process of decomposition starts with complex plant
detritus and other organic matter producing carbon gases and
humus (Figure 2); [15]. The process can efficiently be characterized
by the rate of mass loss, and rates of nutrient immobilization
and release into the soil. The decomposition process can be
distinguished in three phases: early, late and humus-near stage. In
the early stage, decomposition of soluble, unshielded cellulose and
hemicelluloses takes place. The process is influenced by climate. In
the late stage, the influence of climate on decomposition gradually
decreases to essentially zero. Also, negative influence of N on lignin
degradation may come into effect through a repression of de novo
ligninase synthesis. In final, humus-near stage, the decomposition
reaches a limit value. From the very onset of decomposition, the
concentrations of Nitrogen and lignin increase (Figure 3); [16].
Soil microorganisms act as decomposers and indicators of
soil quality by regulating the key process of soil carbon cycling,
including lignin and cellulose degradation and soil carbon turnover
Lei et al. [17]; Li et al. [18]; Sun et al. [19] and Mele [20]. Complex
compounds such as lignin are degraded by enzyme oxidases, which
are produced primarily by fungi while cellulose is degraded by
hydrolases, produced primarily by bacteria in the soil You et al.
[21]. The decomposition of litter and deadwood is a key step in
biogeochemical cycling of carbon and nitrogen as it contributes to
CO2 release Van Geffen et al. [22]. Litter and wood decomposition is
considered to be mainly carried out by fungi and, to a lesser extent,
by bacteria Allmer et al. [23]; Baldrian [24]; Bässler et al. [25];
Purahong et al. [26]; Tlaskal et al. [27]. However, bacteria are able
to degrade lignin and catabolize side products being derived from
incomplete degradation of litter by fungi Bugg et al. [28,29].
Decomposition proceeds through several steps whereby
bacterial community shows a succession of colonizers with most
abundant taxa of Proteobacteria, Actinobacteria and Bacteroidetes
Purahong et al. [26], Tlaskal et al. [27]; Urbanová et al. [30] over the
entire stages of litter decomposition. Similarly, fungal communities
also undergo a clear succession process of different taxa which are
able to decompose the available biopolymers Fukasawa et al. [31];
Purahong et al. [26], Voriskova [32]. Decomposition stage, type of
soil organic matter or quality of litter (lignin or cellulose based),
substrate size, physico-chemical properties of soil, soil structure
and climate parameters control the decomposition process
and hence, play a crucial role in determining the structure and
composition of microbial community (bacteria and fungi) and most
importantly the decay pattern of organic matter.
Global climate change is likely to intensify the decomposition
rates when long-term warming occurs in the absence of moisture
constraints. To understand the responses of ecosystems to future
climate change in terms of shifts in biodiversity and biogeochemical
cycling, it is crucial to understand and quantify the underlying
controls on organic matter decomposition Salinas et al. [33].
Figure 2: a) Generalized pathways for transformation of litter to humus and inorganic C.
b) Stages in litter decomposition Berg and McClaugherty [15].
The + and - signs indicate positively and negatively related effects, respectively, to increased concentrations of nutrients and lignin.
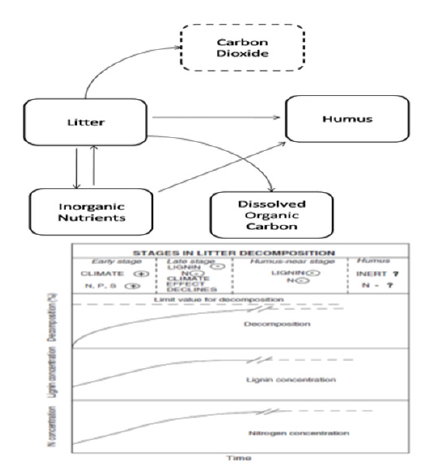
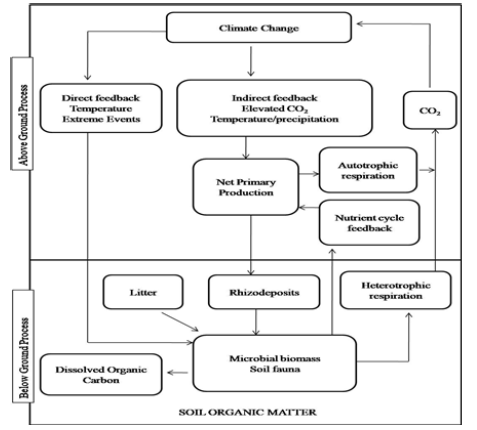
Figure 3: Direct and Indirect effects of climate changes on soil microbial communities and feedback to global warming through carbon dioxide production (Based on Bardgett et al. [16].
Soil Respiration
Soil respiration is the production of CO2 from the soil when
plant roots, microbes, and fauna respire. It is the second largest
carbon flux between the land and atmosphere Raich [34]; Zhao et
al. [35]. The largest share of these fluxes comes from humid tropical
forest. It has been suggested that an increase of 3% in a tropical soil
respiration of 1500gCm-2 per year is greater than a 20% increase in
a tundra soil-respiration rate of 200gCm-2 per year Raich et al. [36].
Soil respiration is one of the largest elements of carbon cycling
in forests Hashimoto et al. [37], main component of ecosystem
respiration Granier et al. [38]; Janssens et al. [39], an important
aspect of soil quality and an indicator of soil fertility Staben et
al. [40]. These facts emphasize the accurate measurement of soil
respiration as an essential component for understanding the carbon
cycle in any ecosystems since small changes in soil respiration may
strongly affect soil carbon sequestration. The rate of soil respiration
is affected by many factors such as soil moisture and temperature,
physico chemical properties of soil, soil microbial composition,
vegetation composition and site productivity. For instance, in case
of sufficient water availability, higher temperatures lead to faster
decomposition of soil organic matter, less storage of carbon in
the slow and passive pools, and greater loss of carbon through
respiration Canadell et al. [41]. The type of vegetation alters the
rate of soil respiration by influencing the quantity and quality of
litter input into the soil that causes variations in soil metabolism.
Climatic factors such as temperature and rainfall also influence
the rates of soil respiration. Soil respiration increases quickly
following rain events in dry climates; however, temperature
sensitivity of soil respiration helps in describing the changes in
CO2 flux with respect to change in temperature Arora [42]. Soil
respiration is often considered as the measure of total microbial
activity in soil. Many studies have reported a significant correlation
between soil respiration and soil microbial biomass carbon pool
Dube et al. [43]; Iqbal et al. [44]; Wang et al. [45]. It is an important
indicator of soil health because it represents the diversity of
organisms occupying microhabitats throughout the soil profile,
level of microbial activity, SOM content and its decomposition.
These microbial mediated decomposition and respiration
pathways act as positive feedback to global warming and may also
in turn be accelerated as a response to increase in atmospheric
temperature. Since, soil respiration is considered to be the sum of
heterotrophic and autotrophic respiration, the combined effect of
microclimatic factors and anthropogenic activities can be modeled
to advance the understanding of the concept. The merits of these
estimations will be helpful in reflecting the important soil-toatmosphere
CO2 efflux Chaudhry [42]. Increased flux of carbon
to roots and soil due to elevated carbon dioxide can stimulate
microbial activity. This stimulation intensifies the degradation of
organic matter, thereby leading to carbon loss from soil.
Mitigation of Green House Gases
There are innumerable ways that soil microbes and their
metabolic activity can affect climate change through land–
atmosphere carbon exchanges. These can broadly be divided into
two categories
a) the ways that affect ecosystem carbon dioxide and methane
uptake, and
b) the ways that control carbon loss from soil through respiration
and production of methane.
Also, changing climate influences soil microbial activity.
Climate change has both direct and indirect effects on the activities
of soil microbes. Direct effects include the influence on soil
microbes, greenhouse gas production under variable conditions of
temperature, precipitation and extreme climatic events. Indirect
effects are climate-driven changes in plant productivity and
diversity which alter soil physicochemical characteristics, carbon
supply to soil, structure and activity of microbial communities
involved in decomposition processes and carbon release from soil
(Figure 3); [16].
The three most important greenhouse gases, CO2, CH4 and
N2O occur naturally in the atmosphere and are produced in
soils, sediments and waters through various microbiological
processes. But due to anthropogenic developmental activities,
the concentration of GHGs has increased substantially in the
atmosphere resulting in accelerated climate warming. Microbial
mediated mitigation of carbon dioxide can well be understood
through the processes of organic matter decomposition and soil
respiration as discussed earlier.
The major source of Methane is landfills where it is produced
through the decomposition of organic wastes. Soil microbial activity
acts as a sink for methane gas. The process follows the oxidation of CH4
into other forms of carbon by a class of microorganisms specifically
bacteria known as methanotrophs. These methanotrophs utilize
CH4 as its sole carbon and energy source in the presence of O2. It
has been estimated that methanotrophic bacteria oxidize 10 to 100
% of the CH4 generated in landfills Borjesson et al. [46]; Chanton &
Liptay [47]; Chanton et al. [48]; Czepiel et al. [49]; Liptay et al. [50]
and Whalen et al. [51] making landfills to act as sink of CH4 rather
than as its source Bogner et al. [52,53]. Hence, it can be inferred
that stimulating the activity of these bacteria in landfill cover soils
could possibly reduce emission of CH4 from landfills.
The stimulation of methane oxidation can be achieved through
the application of nitrogen-based fertilizers Bodelier et al. [54]; De
Visscher et al. [55], Mohanty et al. [56] as the nitrogenous fertilizers
are used as nitrogen sources by the soil microorganisms. However,
the drawback of this application is the production of yet another
greenhouse gas, N2O. Methane-consuming microorganisms are
actually capable of extracting atmospheric methane even at very
low concentrations Zimmerman [57]. These microorganisms are
found both in soil and also in aquatic habitats and at home. Hence,
microbes contribute toward controlling methane emission which in
turn regulates climate change.
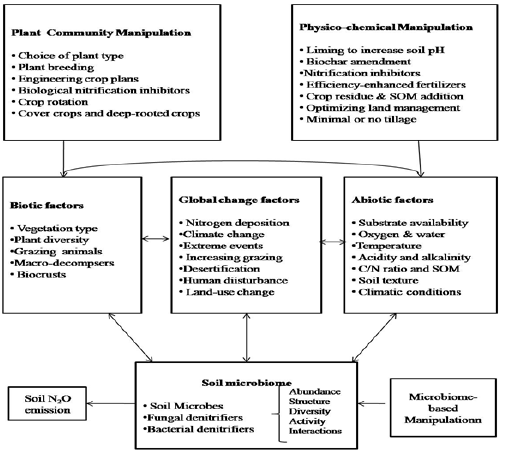
Bacterial and fungal-mediated nitrification and denitrification
are the key processes that account for approximately two-third
of total N2O emissions from the soil predominantly due to the
application of nitrogenous fertilizers Zhu et al. [58]. The abundance,
diversity, community structure and biological pathways of the soil
microbial communities (including ammonia oxidizers, bacterial and
fungal denitrifiers) are affected by a wide range of biotic, abiotic
and emerging global change factors and their interactions. Hence,
any strategy that can enhance the plant capacity to readily uptake
nitrogen from the soil and making it unavailable to microorganisms
can substantially add to the mitigation of soil N2O emissions. The
aim can be achieved through the development of three categories
of technologies
a. physicochemical technologies (by manipulating soil abiotic
factors),
b. plant community-based technologies (by manipulating soil
biotic factors) and
c. microbiome-based technologies (by manipulating soil
microbiome in situ) for future mitigation of climate change
(Figure 4); [59].
Figure 4: The relationships and interactions among N-cycling microorganisms, biotic factors, abiotic factors, global change factors, mitigation strategies and dryland N2O emissions Redrwan from Hu et al. [61].
Conclusion
Climate change and warming have direct and indirect
consequences on soil microbial communities Castro et al. [60]
through modifications in various factors simultaneously. Such
immense alterations can have considerable impacts on the soil
microbiome as well as on plants and ultimately soil carbon balance.
Soil microorganisms contribute significantly to the production and
consumption of greenhouse gases, such as carbon dioxide (CO2),
methane (CH4), nitrous oxide (N2O), and nitric oxide (NO). This
contribution towards production of GHGs by microbes is further
stimulated by anthropogenic activities such as waste disposal and
intensive agriculture. The feedback responses of soil microbes to
increasing GHGs concentration in atmosphere can accelerate or
slow down global warming up to different unknown extent.
Hence, an understanding of the role of soil microbes as both
contributors to and reactive components of climate change can help
us determine whether they can be used to mitigate the emissions
of GHGs or they will accelerate the speed of climate disaster at the
macroscopic and even the global scale. Although various ecosystem
specifically agriculture sector where productivity is very much
dependent on soil microbial activity has been predicted to combat
the adverse impacts of climate change till now, but in near future
this situation may get reversed. Arora et al. [61]. This necessitates
the implementation of appropriate measures to deal with the
problems of changing and climate and their aftereffects on soil
microbiome.
Microbes are involved in many processes, such as the carbon
and nitrogen cycles, and are responsible for both the production
and consumption of greenhouse gases such as carbon dioxide
and methane. Microbes could have various positive and negative
feedback responses to temperature, but the extent of these is not
completely understood. The reason is that microbes live in very
diverse communities that interact with other organisms and the
environment in complex ways, which makes it difficult to make
predictions about the effects of microbes on climate change, but
scientists are trying to include microbial activity in climate change
models. What is certain is that human activities have helped to
increase the production of greenhouse gases by microbes.
Microorganisms have been changing the climate, and have
been changed by the climate, throughout Earth’s history. As we
experience unprecedented environmental impacts from climate
change, microorganisms will respond, adapt, and evolve in their
surroundings. Because they have generation times as short
as a few hours, they will do so at higher rates than most other
organisms. This makes microbes ideal sentinels for understanding
the effects of climate change on biological systems and the global
biogeochemical cycles that microbes mediate. Scientists can study
the effects of climate change on microbes to both understand and
hopefully predict the future effects of climate change on all forms
of life. This colloquium brought together members of the American
Society for Microbiology and the American Geophysical Union
because understanding climate change impacts requires experts
from many scientific disciplines. The collaboration between
these two societies intermingled scientists knowledgeable about
microbial contributions and responses to climate change across
global settings (terrestrial polar regions; soil, agriculture, and
freshwater; oceans) and able to think broadly about the functions
of microbiomes. Although scientists have been studying microbial
ecosystems for many years, we realize we have much more to learn
and understand about complex and interconnected microbial
functions. The information in this report reflects the current
understanding of microbes and our changing climate, as well as
gaps and priorities for future study.
More than 30 microbiologists from 9 countries have issued
a warning to humanity -- they are calling for the world to stop
ignoring an ‘unseen majority’ in Earth’s biodiversity and ecosystem
when addressing climate change. ‘Scientist’s warning to humanity:
microorganisms and climate change’ was published today in the
journal Nature Reviews Microbiology. Professor Rick Cavicchioli,
microbiologist at the School of Biotechnology and Biomolecular
Sciences at UNSW Sydney, has led the global effort. With their
statement, the researchers are hoping to raise awareness both for
how microbes can influence climate change and how they will be
impacted by it -- calling for including microbes in climate change
research, increasing the use of research involving innovative
technologies, and improving education in classrooms. “Microorganisms,
which include bacteria and viruses, are the lifeforms
that you don’t see on the conservation websites,” says Professor
Cavicchioli.
“They support the existence of all higher lifeforms and are
critically important in regulating climate change.
“However, they are rarely the focus of climate change studies
and not considered in policy development.”
Professor Cavicchioli calls microbes the ‘unseen majority’
of lifeforms on earth, playing critical functions in animal and
human health, agriculture, the global food web and industry. For
example, the Census of Marine Life estimates that 90% of the
ocean’s total biomass is microbial. In our oceans, marine lifeforms
called phytoplankton take light energy from the sun and remove
carbon dioxide from the atmosphere as much as plants. The tiny
phytoplankton form the beginning of the ocean food web, feeding
krill populations that then feed fish, sea birds and large mammals
such as whales.
Sea ice algae thrive in sea ice ‘houses’. If global warming trends
continue, the melting sea ice has a downstream effect on the sea ice
algae, which means a diminished ocean food web. “Climate change
is literally starving ocean life,” says Professor Cavicchioli. Beyond
the ocean, microbes are also critical to terrestrial environments,
agriculture and disease. “In terrestrial environments, microbes
release a range of important greenhouse gases to the atmosphere
(carbon dioxide, methane and nitrous oxide), and climate change
is causing these emissions to increase,” Professor Cavicchioli says.
“Farming ruminant animals releases vast quantities of methane
from the microbes living in their rumen -- so decisions about
global farming practices need to consider these consequences.
“And lastly, climate change worsens the impact of pathogenic
microbes on animals (including humans) and plants -- that’s
because climate change is stressing native life, making it easier
for pathogens to cause disease. “Climate change also expands the
number and geographic range of vectors (such as mosquitos) that
carry pathogens. The end result is the increased spread of disease,
and serious threats to global food supplies.” Greater commitment to
microbe-based research needed. In their statement, the scientists
call on researchers, institutions and governments to commit to
greater microbial recognition to mitigate climate change. “The
statement emphasizes the need to investigate microbial responses
to climate change and to include microbe-based research during the
development of policy and management decisions,” says Professor
Cavicchioli.
Additionally, climate change research that links biological
processes to global geophysical and climate processes should have a
much bigger focus on microbial processes. “This goes to the heart of
climate change, so if micro-organisms aren’t considered effectively,
it means models cannot be generated properly and predictions
could be inaccurate,” says Professor Cavicchioli. “Decisions that
are made now impact on humans and other forms of life, so if you
don’t take into account the microbial world, you’re missing a very
big component of the equation.” Professor Cavicchioli says that
microbiologists are also working on developing resources that
will be made available for teachers to educate students on the
importance of microbes. “If that literacy is there, that means people
will have a much better capacity to engage with things to do with
microbiology and understand the ramifications and importance of
microbes.”
References
- Fierer N, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecology 88(6): 1354-1364.
- Arora P, Chaudhry S (2015) Carbon sequestration potential of populus deltoides plantation under social forestry scheme in Kurukshetra, Haryana in Northern India. Journlal of Materials and Environmental Studies 6(3): 713-720.
- Gouglias C, Joanna MClark, Shaw LJ (2014) The role of soil microbes in the global carbon cycle: Tracking the below-ground microbial processing of plant-derived carbon for manipulating carbon dynamics in agricultural systems. Journal of the Science of Food and Agriculture 94(12):2362-2371.
- Singh BK, Bardgett RD, Smith P, Reay DS (2010) Microorganisms and climate change: Terrestrial feedbacks and mitigation options. Nat Rev Microbiol 8: 779-790.
- Bardgett RD, van der Putten WH (2014) Belowground biodiversity and ecosystem functioning. Nature 515(7528): 505-511.
- Fellbaum CR, Mensah JA, Pfeffer PE, Kiers ET, Bücking H (2012) The role of carbon in fungal nutrient uptake and transport Implications for resource exchange in the arbuscular mycorrhizal symbiosis. Plant Signal Behav 7(11): 1509-1512.
- Mellado-Vazquez PG, Lange M, Glexiner G (2019) Soil microbial communities and their carbon assimilation are affected by soil properties and season but not by plants differing in their photosynthetic pathways (C3 C4). Biogeochemistry 142: 175-187.
- Liang C, Schimel JP, Jastrow JD (2017) The importance of anabolism in microbi,al control over soil carbon storage. Nat Microbiol 2(8): 17105.
- Cotrufo MF, Soong JL, Horton AJ, Campbell EE, Haddix ML, et al. (2015) Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat Geosci 8(10): 776-779.
- Lange M, Eisenhauer N, Sierra CA, Bessler H, Engels C, et al. (2015) Plant diversity increases soil microbial activity and soil carbon storage. Nat Commun 6: 6707.
- Balser TC, Firestone MK (2005) Linking microbial community composition and soil processes in a California annual grassland and mixed-conifer forest. Biogeochemistry 73(2): 395-415.
- Gleixner G, Poirier N, Bol R, Balesdent J (2002) Molecular dynamics of organic matter in a cultivated soil. Org Geochem 33(3): 357-366.
- Voroney RP, Heck RJ (2015) The soil habitat. In: Paul EA (Ed.), Soil microbiology, ecology and biochemistry. (4th edn), Academic Press, Boston, USA, pp. 15-39.
- Chen JR, Wang QL, Li M, Liu F, Li W (2016) Does the different photosynthetic pathway of plants. affect soil respiration in a subtropical wetland? Ecol Evol 6(22): 8010-8017.
- Berg B, McClaugherty C (2008) Plant litter: Decomposition, humus formation, carbon sequestration. (2nd edn), Springer, Verlag Berlin Hiedelberg, New York, USA.
- Bardgett RD, Freeman C, Ostle NJ (2008) Microbial contributions to climate change through carbon cycle feedbacks. ISME J 2(8): 805-814.
- Lei L, Xiao W, Zeng L, Zhu J, Huang Z, et al. (2018) Thinning but not understory removal increased heterotrophic respiration and total soil respiration in Pinus massoniana stands. Science of the Total Environment 621: 1360-1369.
- Li L, Zhu-Barker X, Ye R, Doane TA, Horwath WR (2018) Soil microbial biomass size and soil carbon influence the priming effect from carbon inputs depending on nitrogen availability. Soil Biology and Biochemistry 119: 41-49.
- Sun S, Zhao H, Xing F, Bai Z, Gao Y, et al. (2017) Response of soil microbial community structure to increased precipitation and nitrogen addition in a semiarid meadow steppe. European Journal of Soil Science 68(4): 524-536.
- Mele PM, Crowley DE (2008) Application of self-organizing maps for assessing soil biological quality. Agriculture, Ecosystems & Environment 126(3-4): 139-152.
- You Y, Wang J, Huang X, Tang Z, Liu S, et al. (2014) Relating microbial community structure to functioning in forest soil organic carbon transformation and turnover. Ecology and Evolution 4(5): 633-647.
- Van Geffen KG, Poorter L, Sass-Klaassen U, Van Logtestijn RS, Cornelissen JH (2010) The trait contribution to wood decomposition rates of 15 Neotropical tree species. Ecology 91(12): 3686-3697.
- Allmer J, Stenlid J, Dahlberg A (2009) Logging-residue extraction does not reduce the diversity of litter-layer saprotrophic fungi in three Swedish coniferous stands after 25 years. Can J for Res 39(9): 1737-1748.
- Baldrian P (2016) Forest microbiome: Diversity, complexity and dynamics. FEMS Microbiol Rev 41 (2): 109-130.
- Bässler C, Müller J, Dziock F, Brandl R (2010) Effects of resource availability and climate on the diversity of wood-decaying fungi. J Ecol 98(4): 822-832.
- Purahong W, Wubet T, Lentendu G, Schloter M, Pecyna MJ, et al. (2016) Life in leaf litter: novel insights into community dynamics of bacteria and fungi during litter decomposition. Mol Ecol 25(16): 4059-4074.
- Tlaskal V, Voriskova J, Baldrian P (2016) Bacterial succession on decomposing leaf litter exhibits a specific occurrence pattern of cellulolytic taxa and potential decomposers of fungal mycelia. FEMS Microbiol Ecol 92 (11): 1-10.
- Bugg TD, Ahmad M, Hardiman EM, Rahmanpour R (2011a) Pathways for degradation of lignin in bacteria and fungi. Nat Prod Rep 28(12): 1883-1896.
- Bugg TD, Ahmad M, Hardiman EM, Singh R (2011b) The emerging role for bacteria in lignin degradation and bio-product formation. Curr Opin Biotechnol 22(3): 394-400.
- Urbanová M, Šnajdr J, Baldrian P (2015) Composition of fungal and bacterial communities in forest litter and soil is largely determined by dominant trees. Soil Biol Biochem 84: 53-64.
- Fukasawa Y, Osono T, Takeda H (2009) Dynamics of physicochemical properties and occurrence of fungal fruit bodies during decomposition of coarse woody debris of Fagus crenata. Journal of Forest Research 14(1): 20-29.
- Voriskova J, Baldrian P (2013) Fungal community on decomposing leaf litter undergoes rapid successional changes. ISME J 7: 477-486.
- Salinas N, Malhi Y, Meir P, Silman M, Cuesta RR, et al. (2010) The sensitivity of tropical leaf litter decomposition to temperature: Results from a large-scale leaf translocation experiment along an elevation gradient in Peruvian forests. New Phytologist 189(4): 967-977.
- Raich JW, Schlesinger WH (1992) The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus 44 B (2): 81-99.
- Zhao Z, Peng C, Yang Q, Meng F, Song X, et al. (2017) Model prediction of biome-specific global soil respiration from 1960 to 2012. Earth’s Future 5(7): 715-729.
- Raich JW (2017) Temporal variability of soil respiration in experimental tree plantations in lowland Costa Rica. Forests 8(2): 40.
- Hashimoto S, Tanaka N, Suzuki M, Inoue A, Takizawa H, et al. (2004) Soil respiration and soil CO2 concentration in a tropical forest, Thailand. Journal of Forest Research 9(1): 75-79.
- Granier A, Ceschia E, Damesin C, Dufrene E, Epron D, et al. (2000) The carbon balance of a young beech forest. Functional Ecology 14(3): 312-325.
- Janssens IA, Lankreijer H, Matteucci G, Kowalski AS, Buchmann N, et al. (2001) Productivity overshadows temperature in determining soil and ecosystem respiration across European forests. Global Change Biology 7(3): 269-278.
- Staben ML, Bezdicek DF, Smith JL, Fauci MF (1997) Assessment of soil quality in conversation reserve program and wheat–fallow soils. Soil Science Society of America Journal 61(1): 124-130.
- Canadell JG, Kirschbaum M, Kurz WA, Sanz MJ, Schlamadinger B, et al. (2007) Factoring out natural and indirect human effects on terrestrial carbon sources and sinks. Environmental Science and Policy 10: 370-384.
- Arora P, Chaudhry S (2017) Dependency of rate of soil respiration on soil parameters and climatic factors in different tree plantations at Kurukshetra, India. Tropical Ecology 58(3): 573-581.
- Dube F, Zogal E, Stolpe N, Espinosa M (2009) The influence of land use change on the organic carbon distribution and microbial respiration in a volcanic soil of the Chilean Patagonia. Forest Ecology and Management 257(8): 1695-1704.
- Iqbal J, Hu R, Feng M, Lin S, Malghani S, Ali IM (2010) Microbial biomass and dissolved organic carbon and nitrogen strongly affect soil respiration in different land use: A case study at three gorges reservoir area, South China. Agriculture, Ecosystems and Environment 137(3-4): 249-307.
- Wang Q, Xiao F, He T, Wang S (2013) Responses of labile soil organic carbon and enzyme activity in mineral soils to forest conversion in the subtropics. Annals of Forest Science 70(6): 579-587.
- Borjesson G, Chanton J, Svensson BH (2001) Methane oxidation in two Swedish landfill covers measured with carbon-13 to carbon-12 isotope ratios. Journal of Environmental Quality 30(2): 369-376.
- Chanton J, Liptay K (2000) Seasonal variation in methane oxidation in a landfill cover soil as determined by an in situ stable isotope technique. Global Biogeochemical Cycles 14(1): 51-60.
- Chanton JP, Rutkowski CM, Mosher B (1999) Quantifying methane oxidation from landfills using stable isotope analysis of downwind plumes. Environmental Science & Technology 33(21): 3755-3760.
- Czepiel PM, Mosher B, Crill PM, Harriss RC (1996) Quantifying the effect of oxidation on landfill methane emissions. Journal of Geophysical Research-Atmospheres 101:16721-16729.
- Liptay K, Chanton J, Czepiel P, Mosher B (1998) Use of stable isotopes to determine methane oxidation in landfill cover soils. Journal of Geophysical Research-Atmospheres 103(D7): 8243-8250.
- Whalen SC, Reeburgh WS, Sandbeck KA (1990) Rapid methane oxidation in a landfill cover soil. Applied and Environmental Microbiology 56(11): 3405-3411.
- Bogner J, Spokas K, Burton E, Sweeney R, Corona V (1995) Landfills as atmospheric methane sources and sinks. Chemosphere 31(9): 4119-4130.
- Bogner JE, Spokas KA, Burton EA (1997) Kinetics of methane oxidation in a landfill cover soil: Temporal variations, a whole landfill oxidation experiment, and modeling of net CH4 emissions. Environmental Science & Technology 31(9): 2504-2514.
- Bodelier PLE, Hahn AP, Arth IR, Frenzel P (2000) Effects of ammonium-based fertilisation on microbial processes involved in methane emission from soils planted with rice. Biogeochemistry 51: 225-257.
- De Visscher A, Cleemput O (2003) Induction of enhanced CH4 oxidation in soils: NH4 + inhibition patterns. Soil Biology & Biochemistry 35(7): 907-913.
- Mohanty SR, Bodelier PLE, Floris V, Conrad R (2006) Differential effects of nitrogenous fertilizers on methane-consuming microbes in rice field and forest soils. Applied and Environmental Microbiology 72(2): 1346-1354.
- Zimmerman L, Labonte B (2015) Climate change and the microbial methane banquet. Climate Alert 27(1).
- Zhu YG, Wang XH, Yang XR, Xu HJ, Jia Y (2014) Key microbial processes in nitrousoxide emissions of agricultural soil and mitigation strategies (in Chinese). Environ Sci 35(2): 792-800.
- Hu HW, Trivedi P, He JZ, Singh BK (2017) Microbial nitrous oxide emissions in dryland ecosystems: mechanisms, microbiome and mitigation. Environ Microbiol 19(12): 4808-4828.
- Castro HF, Classen AT, Austin EE, Norby RJ, Schadt CW (2010) Soil microbial community responses to multiple experimental climate change drivers. Appl Environ Microbiol 76(4): 999-1007.
- Arora P, Devi R, Chaudhry S (2019) Impact of climate change on the production of major food and commercial crops in India: A five decadal study. International Journal of Environment and Climate Change 9(9): 477-485.
© 2021 Pooja Arora. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






