- Submissions

Full Text
COJ Reviews & Research
Role of Hydration in Biomembranes as a Challenge for Current Biophysics
Disalvo EA*, Cejas JP, Rosa SA and Frias MA
Laboratory of Biointerfaces and Biomimetic Systems, Argentina
*Corresponding author:Disalvo EA, Laboratory of Biointerfaces and Biomimetic Systems, Applied Biophysics and Food Research Center, Argentina
Submission: July 02, 2021; Published: July 12, 2021

ISSN 2639-0590Volum3 Issue2
Abstract
The consideration of water as a structural component gives biological membranes specific thermodynamic properties that explain its responsive behavior when chased by physical and chemical perturbations. These properties are derived from the peculiar surface tension of pure water and its variation due to the presence of hydrogen bonds arrangements between water and membrane components that determine a complex surface free energy profile. A completer and more realistic picture should consider how the hydration properties are linked to mechanical forces with relevance in osmotic stress of cells and in the behavior lipid monolayers. In the present work, the mechano-chemical coupling of surface pressure with water activity in a lipid interphase is explored as a way to mimic the thermodynamic response of the lipid interphase to bioactive compounds. As a result, the relaxation processes during the action of bio effectors are described in terms of water organization around the lipid residues such as acyl chains and polar head groups.
Membrane Models
The classical approaches to understand the properties of a lipid membrane as a thermodynamic system consider it as a close autonomous phase, i.e., its properties are independent of the adjacent media and ignore water as a component [1]. However, lipids are not in vacuum. Due to its unique properties as a structured liquid, lipid aggregates in bilayers sequestering a finite amount of water in its structure. This induces the formation of a region where lipids and water interrelate to give place a region with unique properties in terms of colloid physical chemistry criteria [2]. Water organizes lipids and the presence of lipids induces a water molecules reorganization following patterns different than pure bulk water. This cross relationship determines surface forces intimately linked to membrane response [3]. Thus, the consideration of the organization of water as a structural component of biological membranes has important implications in functional physiological responses considering them under thermodynamic, dynamic and kinetic criteria [4,5]. In particular, surface tension, dielectric permittivity and heat capacity can be named, all of them related to the formation of H bonds between water molecules themselves and with chemical residues along the membrane surface topology [6-9].
The Membrane State
In terms of thermodynamic variables, lipid phases can be changed thermotropically at constant water content or lyotropically i. e. varying water activity at constant temperature. Thus, a state of the membrane can be defined by a water activity/temperature pair. However, membranes are subjected to mechanical stress. In a model system, the immediate example is given by the lipids in a monolayer at different surface pressure. In natural membranes, the coupling with cytoskeleton affects also the lateral pressure of the lipids. This picture is more complex when proteins are involved. This last point raises the question of whether the membrane state can be defined for a membrane in a mechanically isolated system or, on the contrary, its coupling with the cytoplasmic media is a variable to take into account [10,11].
An extension of the view of the membrane as a non-autonomous system admits the correlation of the membrane by means of interfacial phenomena with the cytoplasmic events on one side and on the external perturbants (bio effectors) on the other, both relevant for cell behavior and response [12]. To understand biological response of cells in terms of thermodynamics properties, i.e. the effect of metabolic changes on membrane properties, it is necessary at a first stage, to define how the membrane is an adequate system for the interplay of perturbation-response variables.
Cell Membrane and Compartmentalization
Cell is a compartmentalized system, both structurally and
kinetically speaking. In turn, the cell membrane is a complex system
of lipids and proteins that confine the cell cytoplasm. However,
its properties cannot be limited to its composition but also by its
state in terms of external variables, such as temperature and media
composition. In this regard, the fact that membrane is in contact
with an active cytoplasm, makes those changes in metabolic stages
affect membrane state. This can be long term effects (for instance
the changes in lipid unsaturation during hydric or cold stress) but
also during the metabolic process without affecting membrane
composition. The messenger in this case can be the water exchange
in a dynamic way [12].
Lipids are composed by hydrocarbon tails of different length,
unsaturation and ramification and by different types of head groups.
The hydrocarbon chain regions are mostly indistinguishable
in terms of structure (they are unstructured oily phases) and
the variation of their properties are changes in density due to
temperature and its polar character due to its ability to solubilizes
water. For instance, the polarization of a pure hydrocarbon phase
such as octanol varies with the amount of water dissolved in it [13].
On the other hand, head groups of the phospholipids protrude from
the phosphates by the presence of different polar residues that are
soluble in water by the presence of H- bonds in different topological
arrays such as choline, ethanolamine, serine, glycerol and inositol
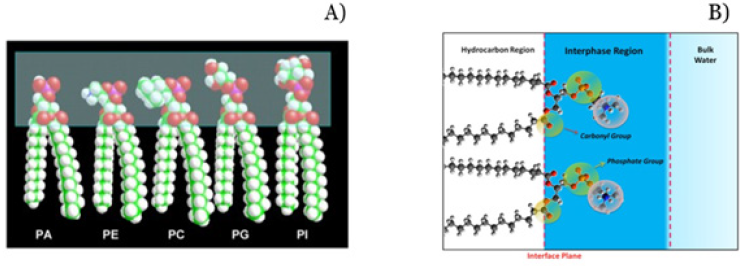
(Figure 1A).
Thus, lipid head groups impose particular (and probably
well defined) water arrays through the polar head groups and its
charges and cannot be ignored in the definition of the system [3].
Energetically, the presence of water populations as independent
or correlated sites of different strength and orientation of H
bonds implies electrical, mechanical, thermal properties of water
rearrangements affecting the interaction of bio effector with the
membrane. The different hydration regions may configure sites
energetically and kinetically distinguishable for compounds´
insertion triggering cooperative and synergistic processes [14].
In this regard, it would be necessary to dilucidate which is
the water organization imposed by the different lipids and which
are thermodynamically significant to give place to membrane
responses and also adaptability.
Figure 1:
A. Different lipids protrude its Polar Part to Water (PA) Phosphatidic Acid, (PE) Phosphatidylethanolamine; (PC) Phosphatidylcholine; (PG) Phosphatidylglycerol (PI) Phosphatidyl Inositol.
B. The Interphase region. Polar groups are imbedded in water. Circles denote strongly bound water and dark blue region the second shell of hydration. Water in between the chains has been avoided for simplicity.
The Membrane Interphase as a Bidimensional Solution
Water organized in layers of around 20A thick at each side of the bilayers conforms an excluded volume that constitutes a barrier for permeant solutes and a repulsion force for peptides and proteins [7,14-18]. This region can be described as a bidimensional solution of hydrated polar groups immersed in water (Figure 1B). In a simplified scheme, the tetrahedral array of bulk water should distort or deform differently according to the nature of the surface next to it.
The protrusion of lipid polar head groups of different nature can bind water molecules in different arrangements. The phosphate and carbonyl groups impose a region in which water binds and organizes in specific patterns by H bonds. Beyond the carbonyl groups water faces nonpolar surfaces in which the strength of H bonds between water molecules are reinforced. These two regions are energetically and entropically different.
Therefore, two kinds of water can be distinguished: hydration water bound to PO2 and CO and loose water in a second hydration shell that penetrates into the first hydrocarbon groups. Due to the different chemical groups protruded to the water phase, it is expected that water would be differently organized in terms of orientation and strength of H bonds and hence energetically different region would be expected. The different groups of the lipid molecules act as hydration sites and organize water in different structures that force new H-bond arrangements, such as phosphate groups in which water molecules should orient with its positive ends (protons), carbonyl groups with electron pairs oriented in 120°, and hydrophobic groups (choline and hydrocarbon chains). This gives place to the surface free energy that drives the insertion processes, i.e., its response to external bio effectors.
Responsive Membrane. A thermodynamic View
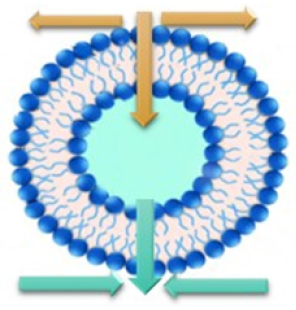
In physical terms, the response is simply the decrease of a free energy, globally or localized. It may have enthalpic and/or entropic contributions. Following these criteria, the interactions of bio effectors with membranes implies water exchange and displacement from the membrane sites to reduce tensions in the hydrogen bond or increase entropy. Concomitant to these changes, membrane parameters as area per lipid and surface density are forced to change. A thermodynamic analysis shows that water exchange can be produced by membrane expansion or compression, due to mechanical forces or osmotic stress [19] (Figure 2).
These processes can be resumed by the following relationship between surface pressure (Δμw ) and water activity (aw) introducing the chemical potential (Δμw) in terms of water activity,
Figure 2: Extrusion (vertical green arrows) and uptake (vertical yellow arrows) of water due to expansion (yellow horizontal lines) or contraction (green horizontal lines) of the lipid membrane. An osmotic balance can trigger expansion (hypotonic media) or contraction (hypertonic media).
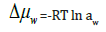
and considering that the diffusion coefficients are related with the friction between the different components (water and lipids)
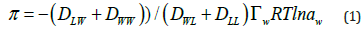
where 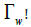 is defined as the surface water concentration.
Diffusion coefficients are: water in water (DWW), lipid in lipids (DLL),
water in lipids (DWL) and lipids in water (DLW).
is defined as the surface water concentration.
Diffusion coefficients are: water in water (DWW), lipid in lipids (DLL),
water in lipids (DWL) and lipids in water (DLW).
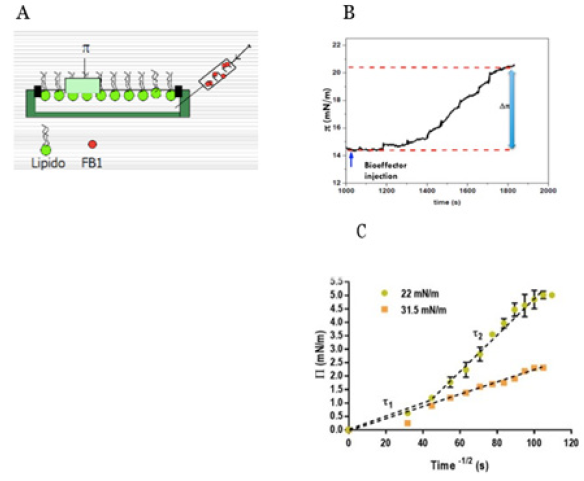
The approach integrating the structural and thermodynamic properties of water provides a mean to understand its response properties. The relevance of the diffusion coefficient is manifested in the process of insertion of hydrophilic or amphyphilic compounds into the membrane. It is possible to measure the change in surface pressure during time after the addition of a bio effector to the subphase of a lipid monolayer (Figure 3A & 3B). In a similar way, the addition in the media of compounds that may permeate or insert in the membrane can be followed by the changes in turbidity caused by the change of the index of refraction of the particle due to changes in its density [20].
The kinetic profile of the processes observed in Figure 3B from an initial state (Πi ) given by a surface pressure/water activity value to a final one ( Π ) can be plotted in terms of the solution of the second Fick’s law (Figure 3C).
The changes in bio effector surface concentration ( Γ ) follows the Ward and Tordai (1) equation:
Figure 3: Kinetics of permeant insertion in monolayers and bilayers.
A. Schematic picture showing the injection of a bio effector in the subphase underneath a lipid monolayer.
B. Kinetic progress of the monolayer surface pressure after the insertion of the bio effector at constant area.
C. Surface pressure square root of time for different initial surface pressures [1].
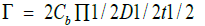
where Cb is the bulk concentration, and D is the diffusion coefficient of the bio effector. The surface pressure can be expressed in terms of interfacial concentration ( Γ ) by
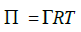
Then,
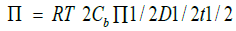
where Π = 3.1416, T is temperature in kelvin and R is the gas constant.
The plot of the kinetics of the process, shown in Figure 3C, gives different profiles according to the initial surface pressure. In fact, the different initial surface pressure determines different initial water activities that affects Γ . The kinetic pattern evolves to a linear one when water activity decreases. It may be concluded that when water is decreased in the membrane by surface pressure the diffusion coefficient does not remain constant. This is probably due to the interplay of the cross-coefficients included in equation 1. Thus, it is likely that relaxation processes implied in the nonlinear response is related to the presence of water and its reorganization. Taken together, the relaxation process can be ascribed to water in the system confined between the lipids. In addition, the changes observed in the refractive index due to hypertonic shrinkage or hypotonic swelling indicates that changes of water after the lipid density. This may be related to the changes of water in between the hydrocarbon chains as denoted elsewhere [21].
Conclusion
Lipid hydration and its effect on the physical chemical properties of lipid membranes has been a matter of discussion in membrane biophysics [21-24]. Lipid hydration is known to strongly affect molecular interactions in the headgroup region, and for that it is determinant of membrane response. The response of the membrane to attain a new equilibrium triggered by a bio effector is governed by a kinetics modulated by the presence of water levels and activities. In this regard, structural changes along the process ultimately related to water lipid arrangements can be speculated. Lipid membranes are important biological matrixes in which biochemical processes take place if they are considered in an integrate way in cell structure. The interdependence of the hydration degree and dynamics of water in cell structures and its participation in osmotic and hydric stresses seems to be fundamental in cell aging and several pathologies. Studies of the behavior of lipid membrane coupled to cytoplasmic-like media are required.
References
- Frias MA, Disalvo EA (2020) Breakdown of classical paradigms in relation to membrane structure and functions. Biochimica et Biophysica Acta -Biomembranes 1863(2): 183512.
- Bagatolli LA, Stock RP (1863) Lipids, membranes, colloids and cells: A long view. Biochimica et Biophysica Acta (BBA) - Biomembranes 1836(10): 183684.
- Alarcón LM, de los Angeles Frías M, Morini MA, Sierra MB, Appignanesi GA, et al. (2016) Water populations in restricted environments of lipid membrane interphases. The European Physical Journal E 39(10): 94.
- Damodaran S (1998) Water activity at interfaces and its role in regulation of interfacial enzymes: A hypothesis. Colloids and Surfaces B: Biointerfaces 11(5): 231-237.
- Disalvo EA, Pinto OA, Martini MF, Bouchet AM, Hollmann A, et al. (2015) Functional role of water in membranes updated: A tribute to Trauble. Biochimica et Biophysica Acta (BBA) - Biomembranes 1848(7): 1552-1562.
- Heimburg T (2008) Thermal biophysics of membranes, John Wiley & Sons, Hoboken, New Jersey, USA.
- Pfeiffer H (2015) Hydration forces between lipid bilayers: A theoretical overview and a look on methods exploring dehydration. Subcell Biochem 71: 69-104.
- Frias L, Disalvo EA (2009) Configuration of carbonyl groups at the lipid interphases of different topological arrangements of lipid dispersions. Langmuir 25(14): 8187- 8191.
- Disalvo E, Frias M (2013) Water state and carbonyl distribution populations in confined regions of lipid bilayers observed by FTIR spectroscopy. Langmuir 29(23): 6969- 6974.
- Evans EA (2018) Mechanics and Thermodynamics of Biomembranes. CRC press, Taylor & Francis Group, Florida, USA.
- Evans E, Needham D (1987) Physical properties of surfactant bilayer membranes: thermal transitions, elasticity, rigidity, cohesion and colloidal interactions. J Phys Chem 91(16): 4219-4228.
- Bagatolli LA, Stock RP, Olsen LF (2019) Coupled response of membrane hydration with oscillating metabolism in live cells: an alternative way to modulate structural aspects of biological membranes? Biomolecules 9(11): 687.
- Pérez HA, Disalvo A, de los Angeles Frías M (2019) Effect of cholesterol on the surface polarity and hydration of lipid interphases as measured by Laurdan fluorescence: new insights, Colloids Surf. B: Biointerfaces 178: 346-351.
- Disalvo EA, Hollmann A, Semorile L, Martini MF (2013) Evaluation of the Defay- Prigogine model for the membrane interphase in relation to biological response in membrane-protein interactions. Biochimica et Biophysica Acta (BBA) - Biomembranes 1828(8): 1834-1839.
- Disalvo E, De Gier J (1983) Contribution of aqueous interphases to the permeability barrier of lipid bilayers for non-electrolytes. Chem Phys Lipids 32(1): 39-47.
- Simon S, Fink C, Kenworthy A, McIntosh T (1991) The hydration pressure between lipid bilayers. Comparison of measurements using x-ray diffraction and calorimetry. Biophys J 59(3): 538-546.
- Le Neveu DM, Rand R (1977) Measurement and modification of forces between lecithin bilayers. Biophys J 18(2): 209-230.
- Marcelja S, Radic N (1976) Repulsion of interfaces due to boundary water. Chem Phys Lett 42(1): 129-130.
- Pinto O, Disalvo E (2019) A new model for lipid monolayer and bilayers based on thermodynamics of irreversible processes. PLoS One 14(4): e0212269.
- Disalvo E (1991) Optical properties of lipid dispersions induced by permeant molecules. Chem Phys Lipids 59(3): 199-206.
- Rosa AS, Disalvo EA, Frias MA (2020) Water behaviour at the phase transition of phospholipid matrixes assesed by FTIR. J Phys Chem B 124 (29): 6236-6244.
- Milhaud J (2004) New insights into water–phospholipid model membrane interactions. Biochimica et Biophysica Acta (BBA) - Biomembranes 1663(1-2): 19-51.
- Binder H (2007) Water near lipid membranes as seen by infrared spectroscopy. Eur Biophys J 36(4-5): 265-279.
- Wennerstrom H, Sparr E (2003) Thermodynamics of membrane lipid hydration. Pure Appl Chem 75(7): 905-912.
© 2021 Disalvo EA. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






