- Submissions

Full Text
COJ Reviews & Research
In vivo Evaluation of Serum Biomarkers after Administration of the Biofield Energy Treated Novel Proprietary Test Formulation on L-NAME and High Fat Diet (HFD)-Induced Cardiovascular Disorders in Sprague Dawley Rats
Mahendra Kumar Trivedi1, Alice Branton1, Dahryn Trivedi1 and Snehais Jana2*
1Trivedi Global, Inc., USA
2Trivedi Science Research Laboratory Pvt. Ltd., India
*Corresponding author:Jana S, Trivedi Science Research Laboratory Pvt. Ltd., Thane (W), Maharashtra, India
Submission: June 23, 2021; Published: July 08, 2021

ISSN 2639-0590Volum3 Issue2
Abstract
The present study evaluated cardiovascular health biomarkers from serum after treatment with the Biofield Energy Treated/Blessed novel Proprietary Test Formulation and Biofield Energy Treatment per se to the animals on NG-Nitro-L-Arginine Methyl Ester Hydrochloride (L-NAME) and High Fat Diet (HFD)- induced cardiovascular model in Sprague Dawley rats using various functional serum biomarkers such as Nitric Oxide (NO), angiotensin-II, homocysteine, adiponectin, Apolipoprotein A1 (APO-A1), and Apo B100 (Protein of LDL) using ELISA assay. The test formulation was formulated including minerals (magnesium, zinc, copper, calcium, selenium, and iron), vitamins (ascorbic acid, pyridoxine HCl, vitamin B9, vitamin B12, and vitamin D3), Cannabidiol (CBD) isolate, Panax ginseng extract, and β-carotene. The constituents of the test formulation were divided into two parts; one part was referred as the untreated test formulation, while the other part and three group of animals received Biofield Energy Healing Treatment/Blessing remotely for about 3 minutes by a renowned spiritual healing expert, Mr. Mahendra Kumar Trivedi. Serum NO was found to be decreased by 12.1%, 22.1%, 25.3%, 28.3%, and 29.1% in the G5 (L-NAME+HFD + the Biofield Energy Treated test formulation), G6 (L-NAME + HFD + Biofield Energy Treatment per se to animals from day -15), G7 (L-NAME+HFD + the Biofield Energy Treated/Blessed test formulation from day -15), G8 (L-NAME+HFD + Biofield Energy Treatment/Blessing per se plus the Biofield Energy Treated/Blessed test formulation from day -15), and G9 (L-NAME+HFD + Biofield Energy Treatment per se animals plus the untreated test formulation) groups, respectively, as compared to the disease control G2 group. However, serum NO was decreased by 9.9% and 10.9% in the G8 and G9 groups, respectively than untreated test formulation (G4) group. In addition, serum angiotensin-II level was significantly decreased by 14.1% in the G6 group as compared to the G4 group. Similarly, the homocysteine level was significantly decreased by 56.36%, 47.84%, 45.93%, 54.85%, and 53.79% in the G5, G6, G7, G8, and G9 groups, respectively as compared to the G2. Adiponectin level was increased by 34.97%, 25.15%, 46.01%, 22.70%, and 38.24% in the G5, G6, G7, G8, and G9 groups, respectively as compared to the G4 group. APO-A1 level in the G7 group showed increased APO-A1 by 13.5% and 10.4% as compared to the G2 and G4 group, respectively. Apo-B100 was significantly decreased by 12.5%, and 13.8% in the G7 and G9 groups, respectively than G4 group. Thus, all serum biomarkers defined as improved tested parameters after treatment with Biofield Energy Treated test formulation and Biofield Energy Treatment per se along with preventive measure on the animals, which could be beneficial against many cardiovascular disorders. Therefore, the results showed the significant reduced antioxidant level, reduced stress-related cardiovascular disease progression and its complications/symptoms such as Ischaemia, myocardial infarction, cardiac and vascular protection, cardiovascular risk factor, stroke, peripheral vessel diseases, and pulmonary embolism, and many cardiovascular health diseases. Biofield Energy Treatment/Blessing might act as a preventive maintenance therapy to maintain good health, to fully restore health, or to improve the overall health and quality of human life.
Keywords: Biofield treatment; Cardiovascular disorders; Myocardial infarction; Adiponectin; Apolipoprotein; The Trivedi effect®; ELISA; High fat diet
Introduction
Cardiovascular disease is one of the significant reasons for high morbidity. Heart related
disorders are due to the increasing interest in the developing countries and their high
experience due to altered lifestyle that causes novel risk factors for cardiovascular disease risk throughout the developing world [1,2]. Cardiac health issues
are related with the various linked pathologies such as Coronary
Heart Disease (CHD), cerebrovascular disease, peripheral arterial
disease, rheumatic, Congenital Heart Diseases (CHD) and venous
thromboembolism. More than 31% mortality worldwide is related
with cardiac disorders and among them most of them are due to
CHD and cerebrovascular accident [3,4]. Various reports suggested
that many cardiac health issues arise within the cardiovascular
system such as endocarditis, rheumatic heart disease, abnormalities
in the conduction system, among others, Cardiovascular Disease
(CVD) or heart disease. World Health Organisation (WHO) reported
that around 75% of premature CVD cases are preventable and risk
factor amelioration could reduce the growing CVD burden on both
individuals and healthcare providers [5].
Various cardiac biomarkers have been reported as an
important factor that suggested change in cardiovascular health
i.e., NO, Angiotensin-II, Homocysteine, Adiponectin, APO-A1, and
Apo B99). It is well established that Nitric Oxide (NO) produced in
vascular endothelial cells has a potent vasodilator effect and plays
an important role in vascular resistance and growth. However,
experimental setup includes administration of L-arginine analogues
such as NG-Nitro-L-Arginine Methyl Ester Hydrochloride (L-NAME)
inhibits Nitric Oxide Synthase (NOS) activity and hence NO
biosynthesis, leading to hypertension [6]. Angiotensin II is a potent
vasoconstrictor and affects cardiovascular homeostasis. Apart
from its role in vasoconstriction, angiotensin II also stimulates the
release of aldosterone, further increasing blood volume and BP due
to water and salt retention. Cardiovascular diseases comprised of
diseases of the heart and blood vessels. Homocysteine is known as
an independent risk factor for atherosclerosis [7]. Arteriosclerosis is
defined as a continuous inflammatory damage to the arterial intima
with increased permeability to plasma, deposition of plasma lipids
in plaques and fibrosis, and calcification of plaques. Homocysteine
has also been positively associated with both diastolic and systolic
blood pressure. Similarly, elevated levels of apolipoprotein (apo)
B, a constituent of atherogenic lipoproteins, and reduced levels
of Apo A-I, a component of anti-atherogenic HDL, are associated
with increased cardiac events [8,9]. Thus, these serum biomarkers
are used to study in presence of NG-Nitro-L-Arginine Methyl
Ester Hydrochloride (L-NAME) and High Fat Diet (HFD)-induced
cardiovascular disorders in Sprague Dawley Rats, a novel test
formulation was designed with the combination of vital minerals
(selenium, zinc, iron, calcium, copper, and magnesium), essential
vitamins (cyanocobalamin, ascorbic acid, pyridoxine HCl, vitamin
B9, and cholecalciferol), and nutraceuticals (β-carotene, Ginseng,
Cannabidiol Isolate (CBD)). All the minerals and vitamins used in
the test formulation have significant functional role to provide vital
physiological effects [10-12]. Besides, cannabidiol itself has wide
range of pharmacological profile and has been reported to role
in different disorders [13,14], while ginseng extract is regarded
as the one of the best immune boosters for overall immunity and
antioxidative activity [15]. In the present study, effect of Biofield
Energy Treatment was determined in serum cardiac biomarker.
The potential of the Biofield Energy Treated Proprietary Test
Formulation and Biofield Energy Treatment per se to the animals
on L-NAME and HFD-induced cardiovascular disorders in Sprague
Dawley rats was evaluated using various functional biomarkers in
serum.
Biofield Energy Healing Treatment, as a Complementary and
Alternative Medicine (CAM) is successfully reported and accepted
by National Center for Complementary/Alternative Medicine
(NCCAM) against various disorders [16-18]. Various CAM therapies
have been accepted by the National Centre of Complementary and
Integrative Health (NCCIH) along with the Biofield Energy Healing,
such as deep breathing, Tai Chi, yoga, therapeutic touch, Reiki,
chiropractic/osteopathic manipulation, relaxation techniques,
pranic healing, meditation, homeopathy, Ayurvedic medicine,
movement therapy, mindfulness, traditional Chinese herbs and
medicines in biological systems, etc. [19-21]. However, impact of
the Trivedi Effect®-Consciousness Energy Healing Treatment is
reported to have beneficial impact on various living and non-living
things. The Trivedi Effect®-Consciousness Energy Healing was
scientifically reported on various disciplines such as in the materials
science [22,23], agriculture science [24], antiaging [25], Gut health
[26], nutraceuticals [27], pharmaceuticals [28], cardiac health [29],
overall human health and wellness. In this study, the authors wish
to study the impact of the Biofield Energy Treatment (the Trivedi
Effect®) on the given novel test formulation and Biofield Energy
Treatment per se to the animals on serum cardiac biomarkers (NO,
Angiotensin-II, Homocysteine, Adiponectin, APO-A1, and Apo B99)
in presence of L-NAME and HFD-induced cardiovascular disorders
in Sprague Dawley Rats using standard ELISA assay.
Material and Methods
Chemicals and reagents
The test formulation components such as pyridoxine hydrochloride (vitamin B6), atorvastatin, zinc chloride, magnesium (II) gluconate, and β-carotene (retinol, provit A) were purchased from TCI, Japan. In addition, copper chloride, cyanocobalamin (vitamin B12), calcium chloride, cholecalciferol (vitamin D3), iron (II) sulfate, captopril, L-NAME, and sodium carboxymethyl cellulose (Na-CMC) were procured from Sigma-Aldrich, USA. Ascorbic acid (vitamin C), vitamin B9 (folic acid), and sodium selenate were obtained from Alfa Aesar, India. However, the cannabidiol isolate and Panax ginseng extract were obtained from Panacea Phytoextracts, India and Standard Hemp Company, USA, respectively. Standard normal chow diet and high fat diet were purchased from Altromin, USA and Research Diets, USA. To estimate the cardiovascular disorder, vital serum biomarkers were evaluated using specific serum biomarker such as NO, Angiotensin-II, Homocysteine, Adiponectin, APO-A1, and Apo-B100 using specific ELISA kits, which were procured from CUSABIO, USA.
Maintenance of animal
Animal cardiovascular activity was performed using randomly breed male Sprague Dawley (SD) rats weighing ranges from 200 to 300gm. All the experimental animals were purchased from M/s. HYLASCO Biotechnology (India) Pvt. Ltd., India. All the animals were handled humanely with due regard for their welfare. The animal care will comply with the regulations of the Committee for the Purpose of Control and Supervision of Experiments on Animals, Ministry of Environment and Forest, Govt. of India. The test facility was registered for animal experiments, while all the animals was procured using the Animal Ethics protocol and the husbandry conditions will be maintained as per the recommendations. Animals were randomly divided into nine groups based on their body weights consist of 10 animals of each group at the time of treatment period. They were kept individually in sterilized polypropylene cages with stainless steel top grill having provision for holding pellet feed and drinking water bottle fitted with stainless steel sipper tube. The animals were maintained as per standard protocol throughout the experiment.
Biofield treatment: consciousness energy healing strategies
The novel test formulation ingredients were divided into two parts. One part of the test compound did not receive any sort of treatment and were defined as the untreated or control sample. The second part of the test formulation was treated with the Trivedi Effect®-Energy of Consciousness Healing Treatment/Blessing (Biofield Energy Treatment) by a renowned Biofield Energy Healer, Mr. Mahendra Kumar Trivedi under laboratory conditions for about 3 minutes. Besides, three group of animals also received Biofield Energy Healing Treatment (known as the Trivedi Effect®) by Mr. Mahendra Kumar Trivedi under similar laboratory conditions for about 3 minutes. The Biofield Energy Healer was located in the USA; however, the test formulation was located in the research laboratory of Dabur Research Foundation, New Delhi, India. The Biofield Energy Healing Treatment/Blessing (prayer) was done remotely, for about 3 minutes via online web-conferencing platform. After that, the Biofield Energy Treated/Blessed samples was kept in the similar sealed condition and used as per the study plan. In the same manner, the control test formulation group was subjected to “sham” healer for about 3 minutes treatment, under the same laboratory conditions. The “sham” healer did not have any knowledge about the Biofield Energy Treatment. The Biofield Energy Treated animals and the formulation constituents were taken back to the experimental room for further experimental proceedings.
Experimental details
The animals were randomized seven days after acclimatization and grouped based on the body weight. The test formulation was prepared freshly prior to dosing and administered to the animals using an oral intubation needle attached to an appropriately graduated disposable syringe. The dose volume was given to the animals 10mL/kg in morning and evening as per body weight. The experimental groups were divided as G1 as normal control (vehicle, 0.5% w/v CMC-Na); G2 as disease control (L-NAME + HFD + 0.5% CMC); G3 as reference item (L-NAME + HFD + Captopril + Atorvastatin); G4 includes L-NAME + HFD along with untreated test formulation; G5 as L-NAME + HFD + Biofield Energy Treated test formulation; G6 group includes L-NAME + HFD + Biofield Energy Treatment per se to animals from day -15; G7 as L-NAME + HFD + Biofield Energy Treated test formulation from day -15; G8 group includes L-NAME + HFD along with Biofield Energy Treatment per se plus the Biofield Energy Treated test formulation from day -15, and G9 group denoted L-NAME + HFD along with Biofield Energy Treatment per se animals plus the untreated test formulation. The normal control animals’ group (G1) was receiving normal drinking water and a normal diet throughout the experimental period. The animals in groups G2-G9 were received L-NAME (20mg/kg, i.p.) and a High Fat Diet (HFD) throughout the experimental period. At terminal (8 weeks treatment), the animals were sacrifice and serum was collected, which was subjected for the estimation of serum biomarkers using suitable ELISA method.
Estimation of NO, Angiotensin-II, Homocysteine, Adiponectin, APO-A1 (Apolipoprotein A1), and Apo B100 in Serum
All the serum biomarkers, which are related to cardiovascular health such as NO, angiotensin-II, homocysteine, adiponectin, and APO-A1 (Apolipoprotein A1) were determined using ELISA Kit using rat’s blood serum as per the manufacturer’s instructions. This technique is a quantitative method, and the principle is based on the binding of antigen and antibody in a sandwich pattern.
Statistical analysis
The data were represented as mean ± Standard Error of Mean (SEM) and subjected to statistical analysis using Sigma-Plot statistical software (Version 11.0). For multiple comparison Oneway analysis of variance (ANOVA) followed by post-hoc analysis by Dunnett’s test and for between two groups comparison Student’s t-test was performed. The p≤0.05 (n=10) was considered as statistically significant.
Results and Discussion
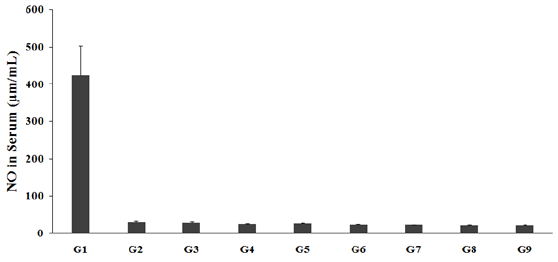
Estimation of Serum Nitric Oxide (NO)
Serum NO was measured after treatment with the test formulation, and the data are presented in Figure 1. The serum NO level in the disease control (L-NAME + high fat diet (HFD) + 0.5% CMC) group (G2) was 30.43±1.94μm/mL, which was significantly decreased as compared with the normal control (G1, 424.30± 79.03μm/mL). Moreover, the positive control (captopril + atorvastatin) treatment (G3) showed the slight decreased by 6.7%. The serum NO was significantly decreased by 2.1%, 6.2%, 9.9%, and 10.9% in the G6 (L-NAME + HFD + Biofield Energy Treatment per se to animals from day -15), G7 (L-NAME+HFD + the Biofield Energy Treated test formulation from day -15), G8 (L-NAME + HFD + Biofield Energy Treatment per se plus the Biofield Energy Treated test formulation from day -15), and G9 (L-NAME + HFD + Biofield Energy Treatment per se animals plus the untreated test formulation) groups, respectively, as compared to the G4, untreated test formulation. In addition, serum NO was reduced by 12.1%, 22.1%, 25.3%, 28.3%, and 29.1% in the G5, G6, G7, G8, and G9 groups, respectively as compared with the G2 group. However, serum NO in G5 (L-NAME + HFD + the Biofield Energy Treated test formulation) was increased by 10.5% as compared to the disease control group (G2) (Figure 1). In serum NO, group G2 (L-NAME+HFD) showed a significant increase as compared to the group G1. However, all the treatment groups (G4, G5, G6, G7, G8, and G9) showed significant decrease in the serum NO levels as compared to the G2. NO, is the hypertension and cardiovascular risk biomarkers, which was reported as one of the vital predictive biomarkers for critical ischaemia duration detection [30]. Besides, serum NO is also regarded as one of the best biomarkers for risk of myocardial infarction in thalassemia [31]. Therefore, serum NO plays a vital role in regulation of a proper cardiac health metabolism and vascular protection. Overall, in this experiment the Biofield Energy Treated/Blessed test formulation and Biofield Energy Treatment per se significantly balancing the serum NO, which might be helpful for the management of cardiovascular disorders. Thus, Mr. Trivedi’s Biofield Therapy would be one of the best alternative treatment approaches for stress-induced cardiovascular dysfunctions.
G1 as normal control (vehicle, 0.5% w/v CMC-Na); G2 as disease control (L-NAME + high fat diet (HFD) + 0.5% CMC); G3 as reference item (L-NAME + HFD + Captopril + Atorvastatin); G4 includes L-NAME + HFD along with untreated test formulation; G5 as L-NAME + HFD along with the Biofield Energy Treated test formulation; G6 group includes L-NAME + HFD along with Biofield Energy Treatment per se to animals from day -15; G7 as L-NAME + HFD along with the Biofield Energy Treated test formulation from day -15; G8 group includes L-NAME + HFD + Biofield Energy Treatment per se + Biofield Energy Treated test formulation from day -15, and G9 group denoted L-NAME + HFD + Biofield Energy Treatment per se animals + untreated test formulation from day -15. The data in bar graph are shown as mean ± SEM (n=10).
Figure 1: Expression of serum nitric oxide (NO) after administration of the biofield treated test formulation and biofield energy healing/blessing on Sprague Dawley rats.
Estimation of serum angiotensin-II
Expression of serum angiotensin-II after administration of Biofield Treated test formulation and Biofield Healing/Blessing per se to the Sprague Dawley rats, and the data is shown in Figure 2. The disease control (L-NAME + high fat diet (HFD) + 0.5% CMC) group (G2) showed the value of serum angiotensin-II as 63.47 ± 1.93pg/ mL, which was increased by 213% as compared with the normal control (G1, 20.28 ±0.8pg/mL) group. However, positive control (captopril + atorvastatin) treatment group (G3) showed decreased level of serum angiotensin-II i.e., 26.05±2.44pg/mL as compared to the G2 group. The level of serum angiotensin-II was decreased by 3.9%, 14.1%, 6.3%, and 6.7% in the G5, G6, G7, and G9 groups, respectively as compared to the untreated test formulation (G4) group (Figure 2). Angiotensin II is regarded as one of the important cardiovascular risk factors, which activates renin-angiotensin system that could damage the heart [32,33]. Overall, Biofield Energy Treatment per se increased the cardiac health possibly by improving the renin-angiotensin system activation that could be beneficial in the cardiovascular patients.
Figure 2: Expression of serum angiotensin-II after administration of biofield treated test formulation and biofield healing/blessing per se to the Sprague Dawley rats.

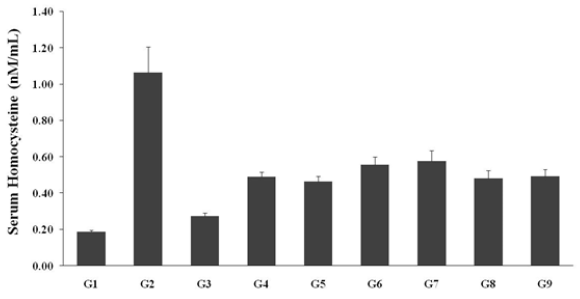
Estimation of serum homocysteine
The level of serum homocysteine was measured after administration of Biofield Treated test formulation and Biofield Healing/Blessing per se and the data are graphically presented in Figure 3. The disease control group (L-NAME + high fat diet, HFD + 0.5% CMC) (G2) showed the value of homocysteine as 1.06±0.14nM/mL, which was increased by 467.33% as compared with the normal control (G1, 0.19±0.01nM/mL) group. While the positive control (captopril + atorvastatin) treatment group (G3) decreased by 74% i.e., 0.27±0.02nM/mL as compared to the G2 group. The level of homocysteine was significantly decreased by 56.36%, 47.84%, 45.93%, 54.85%, and 53.79% in the G5, G6, G7, G8, and G9 groups, respectively, as compared to the disease control group (G2) (Figure 3). However, serum homocysteine level was reduced by 5.29% and 2.02% in the G5 and G8 groups, respectively with reference to G4 group. Various studies suggested that increased serum homocysteine would aggravates cardiac diseases such as impending myocardial infarction, stroke, peripheral vessel diseases, and pulmonary embolism, and thus considered as a potential marker in cardiovascular diseases [34,35]. Thus, Biofield Energy Treatment significantly reduced the serum homocysteine, which might be significantly beneficial for cardiac disorders.
Figure 3: Expression of serum homocysteine after administration of biofield Treated test formulation and biofield healing/blessing per se to the Sprague Dawley rats.
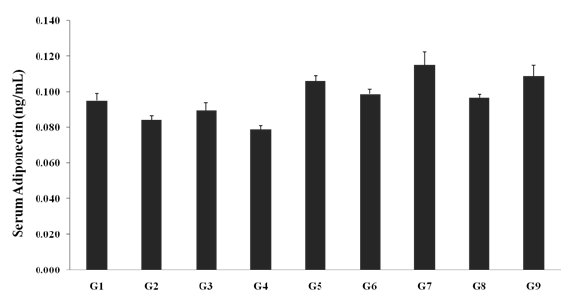
Estimation of adiponectin in serum
Expression of serum adiponectin after administration of Biofield Treated test formulation and Biofield Healing/Blessing per se to the Sprague Dawley rats, and the results are shown in Figure 4. The disease control (L-NAME + high fat diet, HFD + 0.5% CMC) group (G2) showed value of adiponectin in serum as 0.084 ± 0.003ng/ mL (decreased by 11.53%), while it was found as 0.090±0.004ng/ mL in the positive control (captopril + atorvastatin) treatment (G3) group (increased by 6.73% as compared with the G2). The level of adiponectin in serum was increased by 34.97%, 25.15%, 46.01%, 22.70%, and 38.24% in the G5, G6, G7, G8, and G9 groups, respectively with reference to the untreated test formulation (G4) group (Figure 4). Adiponectin and cardiovascular health are the major factors in many disorders. Adiponectin is the adipokines, which possesses multiple salutary action on the insulin sensitivity and cardiovascular health. Further, it protects many cardiovascular health diseases such as vasodilator, anti-inflammatory, antiapoptotic, and anti-oxidative activities in both cardiac and vascular cells [36,37]. However, in this experiment the outcomes revealed that the Biofield Energy Treated test formulation and Biofield Energy Treatment per se to the animals directly regulates the level of adiponectin in serum, which could be improved the cardiovascular performance.
Figure 4: Expression of serum adiponectin after administration of biofield treated test formulation and biofield healing/blessing per se to the Sprague Dawley rats.
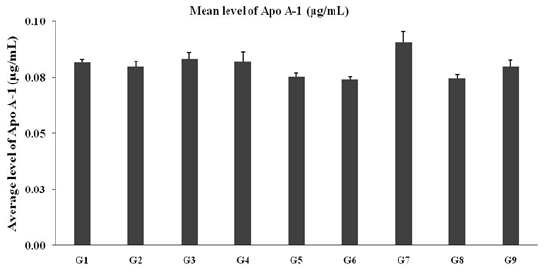
Estimation of serum Apolipoprotein-A1 (Apo-A1)
Expression of Apo-A1 in serum after administration of Biofield Treated test formulation and Biofield Healing/Blessing per se to the Sprague Dawley rats and the data are shown in Figure 5. The level of APO-A1 in the disease control (L-NAME + high fat diet, HFD + 0.5% CMC) group (G2) was 0.080 ± 0.003 16.95μg/mL, while in the positive control (captopril + atorvastatin) treatment (G3) showed increased to 0.083±0.003μg/mL. G7 (L-NAME + HFD + the Biofield Energy Treated test formulation from day -15) group showed increased level of APO-A1 by 13.5% and 10.4% in the as compared to the G2 and untreated test formulation (G4) group (Figure 5) respectively. Serum Apo A-1 has been reported as one of the major biomarkers of coronary artery disease (CAD) [38]. Taking into consideration, CAD being as one of the leading causes of morbidity and mortality worldwide, which is an important public health concern [39]. Overall, the Biofield Energy Treated test formulation and Biofield per se regulates the level of serum Apo A-1, which could be improved the CAD and associated physiological functions.
Figure 5: Expression of Apo-A1 in serum after administration of biofield treated test formulation and biofield healing/blessing per se to the Sprague Dawley rats.
Estimation of Apo-B100 in serum
Serum expression of Apo-B100 after treatment with the Biofield Energy Treated test formulation and Biofield Energy Treatment per se to the animals is shown in Figure 6. The level of Apo-B100 in the disease control (L-NAME + high fat diet, HFD + 0.5% CMC) group (G2) was 0.475±0.013μg/mL, increased by 7% as compared with normal control group, while in the positive control (captopril + atorvastatin) treatment (G3) showed decreased to 0.413±0.012μg/ mL (decreased level of Apo-B100 by 12.9%). However, Apo-B100 in serum was significantly decreased by 8.2%, 6.1%, 12.5%, 6.8%, and 13.8% in the G5, G6, G7, G8, and G9 groups, respectively with reference to untreated test formulation (G4) group (Figure 6). Apolipoproteins (Apo-B100) is one of the cardiac biomarkers in many cardiovascular disorders (CVD) [38].
Figure 6: Expression of ApoB-100 in serum after administration of biofield treated test formulation and biofield healing/blessing per se to the Sprague Dawley rats.

Experiment includes four preventive maintenance groups (G6, G7, G8 and G9). The findings showed the significant slowdown of cardiovascular-related symptoms and also reduced the chances of disease susceptibility. Taking everything into account, it suggests that Mr. Trivedi’s Biofield Therapy/Blessing was found to be most effective and benefited to protect from various disorders and simultaneously to improve the overall health and quality of life.
Conclusion
On the basis of experimental parameters, it was observed that the serum cardiovascular biomarkers were significantly improved after treatment. The results showed that serum NO was significantly reduced by 12.1%, 22.1%, 25.3%, 28.3%, and 29.1% in the G5, G6, G7, G8, and G9 groups, respectively as compared to the disease control, G2 group. However, serum NO was decreased by 10.9% in the G9 group as compared with the G4, untreated test group. Similarly, serum angiotensin-II level was decreased by 14.1% in the G6 group as compared to G4 group. Homocysteine level in serum was significantly decreased by 56.36%, 47.84%, 45.93%, 54.85%, and 53.79% in the G5, G6, G7, G8, and G9 groups, respectively, as compared to the G2 group. Moreover, the level of adiponectin in serum was increased by 34.97%, 25.15%, 46.01%, 22.70%, and 38.24% in the G5, G6, G7, G8, and G9 groups, respectively as compared to the G4 group. The level of APO-A1 was increased by 13.5% and 10.4% in the G7 group with reference to the G2 and G4 groups, respectively. Apo-B100 in serum was significantly decreased by 12.5%, and 13.8% in the G7 and G9 groups, respectively with reference to the G4 group. Altogether, the Trivedi Effect® improved biochemical parameters related to cardiovascular disorders, that also affects the antiaging, antistress, and neuroprotective activities in the preventive maintenance groups (G6, G7, G8, and G9) in L-NAME and high fat diet-induced cardiovascular disorders rat model. This would be the helpful to fight against many cardiovascular disease progressions and to improve the overall health and quality of life. Mr. Trivedi’s Biofield Therapy might also reduce the severity of any type of acute/chronic disease (auto-immune related and inflammatory disorders) progression rate. Further, Mr. Trivedi’s Biofield Energy Healing Therapy/ Blessing could be utilized as a CAM approach for the management of multiple disorders viz. rheumatoid arthritis, myasthenia gravis, fibromyalgia, Addison disease, multiple sclerosis, aplastic anemia, psoriasis, Crohn’s disease, vitiligo, ulcerative colitis, alopecia areata, dermatitis, hepatitis, diverticulitis, Parkinson’s, and stroke, many more.
Acknowledgement
The authors are grateful to Dabur Research Foundation, Trivedi Science, Trivedi Global, Inc., and Trivedi Master Wellness for the assistance and support during the work.
References
- Farley A, McLafferty E, Hendry C (2012) The cardiovascular system. Nurs Stand 27(9): 35-39.
- Ruan Y, Guo Y, Zheng Y (2018) Cardiovascular Disease (CVD) and associated risk factors among older adults in six low-and middle-income countries: Results from SAGE wave 1. BMC Public Health 18(1): 778.
- WHO (2016) Cardiovascular diseases (CVDs).
- Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, et al. (2017) Global regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am College Cardiol 70(1): 1-25.
- NICE (2010) cardiovascular disease prevention. NICE Guideline PH25.
- Fujita K, Maeda N, Sonoda M, Koji O, Toshiyuki H, et al. (2008) Adiponectin protects against angiotensin II-induced cardiac fibrosis through activation of PPAR-alpha. Arterioscler Thromb Vasc Biol 28(5): 863-870.
- Guéant-Rodriguez RM, Spada R, Moreno-Garcia M, Guido A, Paolo B, et al. (2011) Homocysteine is a determinant of ApoA-I and both are associated with ankle brachial index, in an ambulatory elderly population. Atherosclerosis 214(2): 480-485.
- Ganguly P, Alam SF (2015) Role of homocysteine in the development of cardiovascular disease. Nutr J 14: 6.
- Yang N, Yao Z, Miao L, Jia L, Xia G, et al. (2016) Homocysteine diminishes apolipoprotein A-I function and expression in patients with hypothyroidism: A cross-sectional study. Lipids Health Dis 15: 123.
- Byrne JH, Voogt M, Turner KM, Eyles DW, McGrath JJ, et al. (2013) The impact of adult vitamin D deficiency on behaviour and brain function in male Sprague-Dawley rats. PLoS One 8(8): e71593.
- Rayman MP (2000) The importance of selenium to human health. Lancet 356(922): 233-241.
- Beard JL, Connor JR (2003) Iron status and neural functioning. Ann Rev Nutr 23: 41-58.
- Peres FF, Lima AC, Hallak JEC, Crippa JA, Silva RH, et al. (2018) Cannabidiol as a promising strategy to treat and prevent movement disorders? Front Pharmacol 9: 482.
- Nagarkatti P, Pandey R, Rieder SA, Hegde VL, Nagarkatti M (2009) Cannabinoids as novel anti-inflammatory drugs. Future Med Chem 1(7): 1333-1349.
- Kang S, Min H (2012) Ginseng, the 'Immunity Boost': The effects of Panax ginseng on immune system. J Ginseng Res 36(4): 354-368.
- Maizes V, Rakel D, Niemiec C (2009) Integrative medicine and patient-centered care. Explore (NY) 5(5): 277-289.
- Bischof M, Del Giudice E (2013) Communication and the emergence of collective behavior in living organisms: A quantum approach. Mol Biol Int, 987549.
- Cassidy CM (2004) What does it mean to practice an energy medicine? J Altern Complement Med 10(1): 79-81.
- Barnes PM, Bloom B, Nahin RL (2008) Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report 12: 1-23.
- Fan K wai (2005) National Center for Complementary and Alternative Medicine Website. J Med Libr Assoc 93(3): 410-412.
- Wisneski L, Anderson L (2009) The Scientific Basis of Integrative Medicine. Evid Based Complement Alternat Medv 2(2): 2005.
- Trivedi MK, Branton A, Trivedi D, Jana S (2021) Effect of consciousness energy healing treatment on the metal profile and properties of tellurium. Eng Technol Open Acc 3(5): 555623.
- Mahendra KT, Alice B, Dahryn T, Snehasis J (2021) Consciousness energy healing treatment impacted the isotopic abundance ratio of 6-Mercaptopurine (6-MP). Nov Appro Drug Des Dev 5(5): 555673.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Morphological characterization, quality, yield and DNA fingerprinting of biofield energy treated alphonso mango (Mangifera Indica L.) Journal of Food and Nutrition Sciences 3: 245-250.
- Trivedi MK, Jana S (2021) Anti-aging activity of biofield energy treated novel proprietary test formulation by assessment of vital biomarkers in Cerebrospinal Fluid (CSF) in Sprague Dawley rats. On J Neur & Br Disord 5(2): 2021.
- Trivedi MK, Jana S (2021) Evaluation of biofield energy healing treatment based proprietary test formulation on gut health potential in colon cancer cell line (HT-29). J Pharmacol Clin Res 8(4): 555743.
- Trivedi MK, Branton A, Trivedi D, Jana S (2021) Isotopic abundance ratio analysis of consciousness energy healing treated folic acid. Food Nutr Current Res 4(2): 290-295.
- Trivedi MK, Branton A, Trivedi D, Jana S (2020) The consciousness energy healing treatment and its impact on the isotopic abundance ratio analysis of flutamide. Drug Des Int Prop Int J 3(5).
- Trivedi MK, Jana S (2019) In vitro assessment of the biofield treated test item on cardiac function using rat cardiomyocytes cell line (H9c2) via multiparametric analysis. Journal of Hypertension and Cardiology 2(4): 1-12.
- Yavuz C, Yazici S, Karahan O, Sinan D, Ahmet C, Orkut G, et al. (2013) Serum nitric oxide level could be a predictive biomarker for detection of critical ischaemia duration. Biomarkers 18(2): 116-120.
- Helmi N, Choudhry H, Qari M, Taha A, Abdulrahman L, et al. (2018) Association of serum asymmetric dimethyl-arginine and troponin I levels as a risk of myocardial infarction in thalassemia. Afr Health Sci 18(3): 720-726.
- Gavras I, Gavras H (2002) Angiotensin II as a cardiovascular risk factor. J Hum Hypertens 16: S2-S6.
- Cesari M, Kritchevsky SB, Atkinson HH (2009) Angiotensin-converting enzyme inhibition and novel cardiovascular risk biomarkers: Results from the trial of angiotensin converting enzyme inhibition and novel cardiovascular risk factors (TRAIN) study. Am Heart J 157(2): 334.e1-e8.
- Alam N, Khan HI, Chowdhury AW (2012) Elevated serum homocysteine level has a positive correlation with serum cardiac troponin I in patients with acute myocardial infarction. Bangladesh Med Res Counc Bull 38(1): 9-13.
- Ganguly P, Alam SF (2015) Role of homocysteine in the development of cardiovascular disease. Nutr J 14: 6.
- Hui X, Lam KS, Vanhoutte PM, Xu A (2012) Adiponectin and cardiovascular health: An update. Br J Pharmacol 165(3): 574-590.
- Eiras S, González-Juanatey JR (2016) Adiponectin as Biomarker in Coronary Artery Disease. In: Patel V, Preedy V (Eds.), Biomarkers in Cardiovascular Disease, Biomarkers in Disease: Methods, Discoveries and Applications, Springer, Dordrecht, Netherlands, pp. 635-651.
- Rahim S, Abdullah HM, Ali Y (2016) Serum Apo A-1 and its role as a biomarker of coronary artery disease (CAD). Cureus 8(12): e941.
- Santulli G (2013) Epidemiology of cardiovascular disease in the 21st century: Updated numbers and updated facts. J Cardiovasc Dis 1: 1-2.
© 2021 Snehais Jana. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






