- Submissions

Full Text
COJ Reviews & Research
Zinc Oxide Nanostructured Material for Sensor Application
KRS Murthy1, Raghu G K2 and Prakash Binnal1*
1Department of Chemical Engineering, India
2Department of Chemistry, India
*Corresponding author:Prakash Binnal, Department of Chemical Engineering, Karnataka, India
Submission: March 11, 2021; Published: April 14, 2021

ISSN 2639-0590Volum3 Issue1
Abstract
In the present project work, efforts have been made to synthesize non noble metal oxide-based nanostructures (ZnO) through hydrothermal method using green chemical plant extract as a reducing agent. The synthesized metal oxide was characterized using various spectroscopic techniques like UVVis, FTIR, XRD, and also electrochemical techniques. Further, the well characterized nanoparticles have been used in the fabrication of thin film electrode on the surface of rigid glassy carbon electrode through simple drop casting method. Finally, the developed sensor electrode was used in the electrochemical sensing of Dopamine as a model system at trace concentration level.
Keywords: ZnO nanostructured material; Sensor; XRD
Introduction
An electrochemical biosensor is a device for the detection of an analyte that combines
biological component with a detector component [1]. It combines a biochemical recognition
(binding element) with signal conversion unit (transducer). The three major components
of electrochemical biosensor are the sensitive biological element, the transducer and the
bio-receptor [2,3]. The sensitive biological element could be nucleic acids, antibodies or
nanomaterials, the transducer which acts as an interface, measuring the physical change
that occurs with the reaction at the bio-receptor and then transform into a easily measurable
electrical output [4,5]. The detector element which senses the signals from the transducer
are passed to an amplifier where they are amplified and analysed [6]. Zinc oxide is one of
the widely studied best metal oxides and it is relatively bio-safe and biocompatible which
is suitable for various sensor applications. The dimensions of ZnO nanostructure can be
engineered to the size of biochemical species being sensed which make ZnO as an excellent
primary transducer for producing sensitive and selective electro signals [7].
The main objectives of the project work were:
1. To develop green chemical synthetic route for the synthesis of Zinc oxide
nanoparticles.
2. To characterize the synthesized ZnO using UV-Vis, IR, XRD and Cyclic Voltammetry
3. To fabricate thin film sensor electrode.
4. 4. To evaluate electro-analytical applicability of the fabricated thin film sensor
towards the detection of dopamine.
Materials and Methods
Preparation of preparation of green chemical plant extract
The leaves of the tree Muntingia Calabura L. were collected (common names: Jamaica cherry, strawberry tree. The leaves were washed with water to clean them. Then the leaves were dried in shade and thoroughly crushed into a fine powder. The fine powder of leaves was taken in a flat-bottomed flask and water was added so to get slurry. This was heated up to a temperature 60 °C on a sand bath to an extent of 2h. The contents were cooled and filtered to get only the liquid extract. This liquid was heated at 60 °C again for 1 hour to get pure extract.
Synthesis of zinc oxide nanoparticles through hydrothermal method
The ZnO nanoparticles are prepared through the following hydrothermal method. Briefly, 4ml of zinc nitrate (precursor) was mixed with 2ml of plant extract to which 40ml water and 5ml PEG were added in such a way that the volume of solution should be 60% of the volume of reactor vessel. The hydrothermal reactor was subjected to heat treatment at 160 °C for 5h. The contents of the reactor were then transferred into a pre-cleaned beaker and taken for centrifugation. The solution was put in centrifugation tubes and centrifugation was carried out in batches of 15 minutes each at 11000rpm. The solid particles retrieved were dried in hot air oven for 5 hours and subsequently calcinated for 2 hours at 400 °C and then for 4 hours at 500 °C. The resulting ZnO nanoparticles were stocked in airtight contained.
Characterization studies
After the synthesis of zinc oxide by solution based hydrothermal
method using green chemical extract, they were well characterized
with the aid using the following techniques in order to obtain
primary information regarding to the formation of zinc oxide
nanoparticles from zinc nitrate precursor. The raw material
weighed depending on respective percentages and then it is mixed
with magnesium phosphate cement slurry (percentage of addition
of binder is varied to analyze better properties), Water is added
(up to 4 to 6wt%) to the mixture and poured inside the mould
of different shapes and allowed for vibration for few min. Due to
vibration aggregates and matrix occupies the shape of the mould
and it is then allowed to cure. After curing, in order to remove the
moisture, it is dried for 5 hours at 110 °C. After drying it is sintered
at temperature of 1300 °C to 1500 °C to provide strength. It is being
tested for analysis of physical, mechanical and thermal properties.
The characterization of ZnO nanoparticles was done by
following methods.
A. X-Ray Diffraction (XRD)
B. UV-Vis spectroscopy (UV)
C. Fourier Transform Infrared Spectroscopy (FT-IR)
D. Cyclic voltammetry (CV)
Result and Discussion
XRD results
The crystalline size and basic properties of the ZnO nanoparticles were studied by using X-ray diffraction technique. The X-ray diffraction pattern of ZnO nanoparticles prepared in the present study and diffraction pattern of standard ZnO are shown in Figure 1a & 1b, respectively.
Figure 1: XRD pattern of synthesized ZnO nanoparticles.
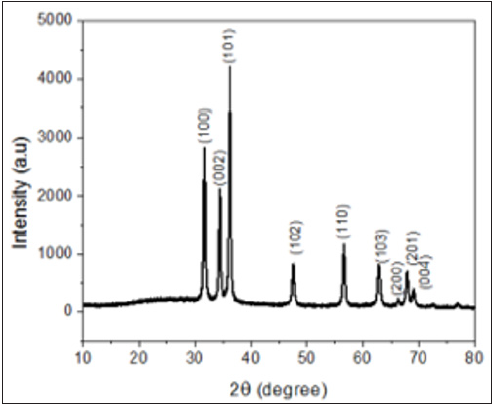
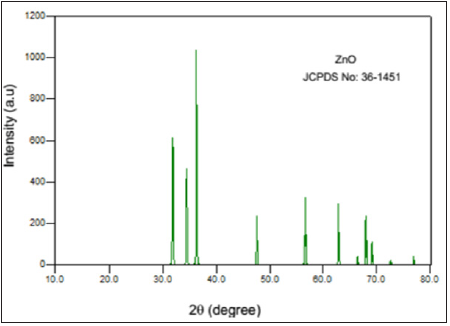
The XRD pattern of ZnO showed various diffraction peaks located at 31, 34.4, 36.1,47, 56, 63, 67, 68, 69 and 72. 2θ values and can be assigned to the (1 0 0), (0 0 2), (1 0 1), (1 0 2), (1 1 0), (1 0 3), (2 0 0), (1 1 2) (2 0 1) and (2 0 4) planes respectively. This result is in good agreement with the JCPDS Card No.36-1451. This confirms the formation of ZnO nanoparticles from the zinc nitrate precursor molecule. The sharp diffraction peaks clearly indicate the as synthesized ZnO are in pure crystalline form and also confirm its hexagonal wurtzite structure [8] (Figure 2).
Figure 2: XRD pattern of ZnO nanoparticles (JCPDS:36-1451).
UV-Vis spectroscopy (UV-Vis)
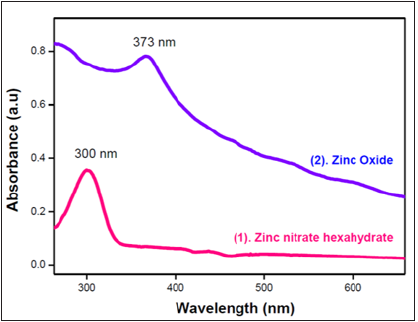
The UV - vis spectrum of zinc nitrate and zinc oxide were recorded in liquid phase and compared in order to confirm the formation of ZnO nanoparticles from zinc nitrate band through blue shift concept and also the nano - dimensionality of ZnO. The samples to record UV-vis spectrum were prepared by dispersing 10 mg/ml zinc oxide and zinc nitrate in water through ultra-sonication and then by transferring it into a cuvette. The absorption spectra of zinc nitrate hexahydrate showed a band at 300nm (Figure 3) whereas the spectrum of zinc oxide (Figure 3) showed an absorption band at 373nm. The band at 373nm is the characteristic for ZnO. This clearly indicates the formation of ZnO from zinc nitrate during hydrothermal process. Further, the shift of band from 300nm to 373nm confirms the nano - dimensionality of ZnO nanoparticles [9].
Figure 3: UV Spectra of synthesized ZnO nanoparticles.
Fourier Transform Infrared Spectroscopy (FTIR)
The FTIR spectra of ZnO nanoparticles have been recorded by using KBr pellet method. The samples were prepared by the hand mixing of IR grade KBr and ZnO in the proportion 1:3. Figure 4 shows the FTIR spectrum of ZnO NPs. The peak at 443cm-1 is assigned to Zn-O stretching vibrational mode of ZnO. The additional peaks observed at 1630cm-1 and 3446cm-1 may be due to O-H bending and stretching frequency of H2O molecules. The peak at 1012cm-1 could be assigned to C-O stretching. Figure 4 shows the FTIR spectrum of zinc nitrate, the peak at the 783cm-1 could be assigned to the absorption of Zn-N. The absence of zinc nitrate in case of IR spectra of ZnO and absence of Zn-O in case of IR spectra of zinc nitrate clearly confirms the formation of zinc oxide nanoparticles [10].
Cyclic voltammetric studies: Sensing of dopamine
Figure 5 shows Cyclic Voltammetric profile of (a) Glassy carbon electrode in the absence of DA, (b) Bare glassy carbon electrode (GC) in presence of DA and (c) Modified glassy carbon electrode in presence of DA. The figure depicts the CV voltammogram of ZnO modified glassy carbon electrode in the absence of dopamine (a), bare glassy carbon electrode (b) and ZnO modified glassy carbon electrode in presence of 10mM of DA in the potential window of -1.0V to 1.0V in a phosphate buffer solution of pH 7.2 at a scan rate of 50 mV/s. The voltammograms recorded for ZnO modified glassy carbon electrode in the absence of DA will not show any voltametric peak in the potential window. This indicates that ZnO has not shown any peaks for the oxidation of zinc into zinc ions. From this we can conclude that ZnO modified glassy carbon electrode can be used as sensor interface in order to study electrolytic behaviour of ZnO with respect to DA analyte molecule. The cyclic voltammogram of 10mM of DA has been recorded at bare glassy carbon electrode in order to distinguish the electrochemical behaviour of DA at bare as well as modified electrode. The cyclic voltammogram recorded at bare glassy carbon electrode has shown an oxidation peak at a peak potential of 0.18V with a peak current of 1.9μA, whereas the modified electrode has shown an oxidation peak at a peak potential 0.22V with a peak current of 3.9μA. These results are in good agreement with literature reports [11]. From this it can be concluded that the use of ZnO nanoparticles synthesized through the hydrothermal method can be used as a suitable candidate in designing of ZnO-nanomaterial dopamine sensors.
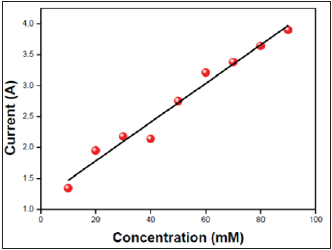
The calibration plot of dopamine sensor is shown in Figure 6. It is constructed by plotting oxidation current measured against the concentration of dopamine for the successive addition of 10mM of dopamine into a 10mL volume of volumetric cell containing phosphate buffer solution of pH 7.4. From the plot it is very clear that the peak current increases linearly with the increase of concentration of dopamine up to 90mM. The developed sensor showed a detection limit of 10μm (3 sigma).
Figure 4: (a) FTIR spectra of Zinc nitrate (b) FTIR spectra of synthesized ZnO nano particles.

Figure 5: Cyclic voltametric profile of a) Glassy carbon electrode and b) Bare glassy electrode
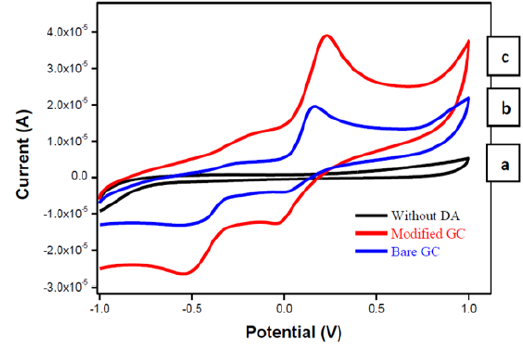
Figure 6: Calibration plot.
References
- Musameh M, Wang J, Merkoci A, Lin Y (2002) Low-potential stable NADH detection at carbon-nanotube-modified glassy carbon electrodes. Electrochem Commun 4(1): 743-746.
- Dequaire M, Degrand C, Limoges B (2000) An electrochemical metalloimmunoassay based on a colloidal gold label. Anal Chem 72 (22): 5521-5528.
- Li H, Eddaoudi M, Richardson DA, Yaghi OM (1998) Porous germanates: Synthesis, structure, and inclusion properties of Ge7O14.5F2[(CH3)2NH2]3(H2O)0.86. J Am Chem Soc 120: 8567-8568.
- Cheng HM, Chiu WH, Lee CH, Tsai SY, Hsieh WF (2008) Formation of branched ZnO nanowires from solvothermal method and dye-sensitized solar cells applications 112(42): 16359-16364.
- Mimani T, Patil KC (2001) solution combustion synthesis of nanoscale oxides and their composites. Mater Phys Mech 4:134-137.
- Shana, Rogers KR (1994) Biosensors. Meas Sci Technol 5(5):461.
- Xu J, Yang H, Fu W, Du K, Sui Y, et al. (2007) Preparation and magnetic properties of magnetite nanoparticles by sol-gel method. J of Magnetism and Magnetic materials 309(2): 307-311.
- Cullit BD, Stock S (2001) Elements of x-ray diffraction, Third edition. Nature 201:167.
- Haiss W, Thanh NTK, Aveyard J, Fernig DG (2007) Determination of size and concentration of gold nanoparticles from UV-vis spectra. Anal Chem 79(11): 4215-4221.
- Daniel MC, Astruc D (2004) Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 104(1): 293-346.
- Dong X, Cao Y, Wang J, Park C, Wang L, et al. (2012) Hybrid structure of zinc oxide nanorods and three-dimensional graphene foam for supercapacitor and electrochemical sensor applications. RSC Advances 2(10): 4364.
© 2021 Prakash Binnal. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






