- Submissions

Full Text
COJ Reviews & Research
Takayasu’s Arteritis: Etiopathogenesis and Treatment Strategies
Paul Gamboa*
Departamento de Intervenciones por Cateterismo, Argentina
*Corresponding author:Paul Gamboa, Departamento de Intervenciones por Cateterismo, Buenos Aires, Argentina
Submission: February 03, 2021; Published: March 19, 2021

ISSN 2639-0590Volum3 Issue1
Generalities
Takayasu’s Arteritis, also known as occlusive thromboaorthopathy, occurs commonly
in young women. It is pulseless vasculitis that essentially affects the aorta and its main
branches, occurring more frequently in young women [1,2]. The term Takayasu disease
was introduced in 1952 by Cacamise et al. [3] in honor to Dr. Mikito Takayasu, professor of
ophthalmology at the University of Kanazawa in Japan, who in 1908 reported on peculiar
arterio-venous anastomoses in the optic disc, caused by retinal ischemia secondary to large
vessel vasculitis in a 21-year-old patient [4,5]. Later, in 1948, Shimizu and Sano detailed the
clinical characteristics of the disease [6].
Although its etiology is not fully elucidated, it is accepted that genetic and infectious factors
may play a role in the pathogenesis. This concept is developed in multiple investigations in
the last decade. It is inferred that Takayasu’s arteritis is an autoimmune disease, in which
cellular immunity plays an important role, but the role of humoral immunity is still unknown.
It is known that the estimulation of an antigen of an unknown nature, possibly infectious
[7], would trigger the expression of heat shock proteins HSP65 (heat shock protein - 65) by
the aortic tissue, activating, at the same time, the major complex class I histocompatibility,
inducing Gamma-delta T cells and natural killer cells to release perforins, which results
in acute inflammation. However, it is necessary to understand the humoral mechanisms
responsible for this vascular damage, in order to create new management strategies, such as
biological agents whose target is cytokines [8,9].
On the other hand, its prevalence varies widely in different geographic regions. For
example, it is one of the main causes of renovascular hypertension in India, Korea, Japan,
China and other countries in Southeast Asia [10]. In contrast, in North America atherosclerosis
and fibromuscular dysplasia are the main cause of renovascular hypertension, that’s why
Takayasu’s arteritis does not occupy a relevant place [11]. This discrepancy in the prevalence
of different geographic areas, supports the idea of a genetic cause. The largest registry
available up to now is in Japan with 5,881 patients affected until 2011, and a prevalence
of 0.004% [12]. It has also been found that the female-male relationship varies in different
countries. In Japan the ratio is 9.4: 1, while in India 1.6: 1, but it preferentially affects the
female gender, which gives rise to a hormonal theory [13]. Although much of the literature
that describes Takayasu’s arteritis has its origin In Asia, in recent decades the recognition of
patients with this disease has increased in Africa, Europe, North America and South America
[14-18].
The natural history of Takayasu’s Arteritis is variable and there is no pathognomonic
sign of the disease. The appearance of inflammatory or systemic characteristics can precede
the vascular symptoms of Takayasu’s Arteritis, delaying its diagnosis for a period of time.
In addition to this, many patients are studied with a diagnosis of fever of unknown origin,
essential hypertension, coarctation, cardiomyopathy, or hypopituitarism before the definitive
diagnosis of Takayasu’s Arteritis [19]. In the acute phase of Takayasu’s Arteritis, there are
nonspecific symptoms such as night sweats, weight loss, and anorexia. While in the chronic
phase the systemic manifestations appear according to the affected organs. Generally,
patients present with claudication (upper limbs 60% vs lower limbs 30%), pulse asymmetry (60 to 80%), and arterial hypertension. Arterial stenosis occurs
three times more than aneurysm. As for them, aneurysms are more
common in the aortic root which can lead to aortic insufficiency.
Cardiac, renal, or central nervous system involvement can increase
the morbidity and disability of the disease [20].
Diagnosis
It is important to remember that an early diagnosis coupled with timely treatment can improve the prognosis of the disease [21]. The average age for diagnosis has been found to be 28.4 years for the black population and 39.3 years for the white population [12]. The clinical characteristics of Takayasu’s Arteritis described by Dr. Numano2 are:
A. Chronic vacuities that affect the intima, media and
adventitia of the ascending aorta, aortic arch and its branches
and the thoracic descending aorta. Most commonly in women
and especially in Southeast Asia
B. Clinical presentation with local pain and signs and
symptoms of regional ischemia due to stenosis or thrombosis.
In rare cases it presents with rupture of vessels. When it wraps
the ascending aorta, it can cause aortic insufficiency.
C. The main cause of death is renovascular hypertension.
D. Erythrocyte sedimentation rate, C-reactive protein and
other inflammation markers are usually elevated. The diagnosis
is confirmed through images.
E. Initial management is with high-dose steroids, maintained
with low-dose steroids and aspirin. In case of poor response,
immunosuppression may be required
In 1988 Ishikawa, based on a series of 96 patients, proposed certain diagnostic criteria which were not widely accepted since it is based on the Japanese population without taking into account the different characteristics that can be found in different populations and the only mandatory criterion was being under 40 years of age, this criterion was its greatest limitation [19] since it is not fulfilled in all cases. In 1990, the American College of Rheumatology defined the diagnostic criteria for Takayasu arteritis (Table 1), being necessary to present three or more of the six criteria, with a specificity sensitivity of 90.5% and 97.8%, respectively [22]. This criterion was based on 63 patients diagnosed with Takayasu arteritis vs 744 patients with vasculitis. Despite being the most accepted criteria in the western world, it has received criticism for not taking into account coronary or pulmonary artery compromise. Furthermore, in countries where the main or only manifestation is involvement of the abdominal aorta, many patients may not be diagnosed according to these criteria.
Table 1:Criteria for the classification of Takayasu Arteritis [ 22].
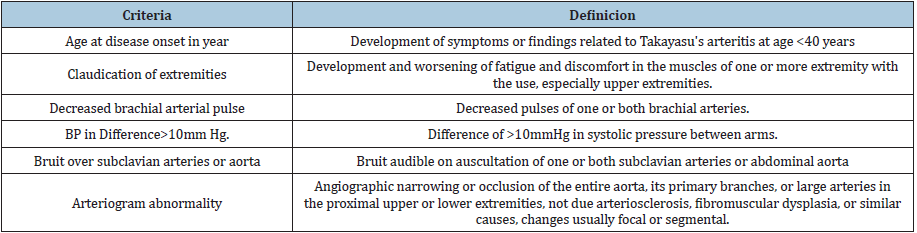
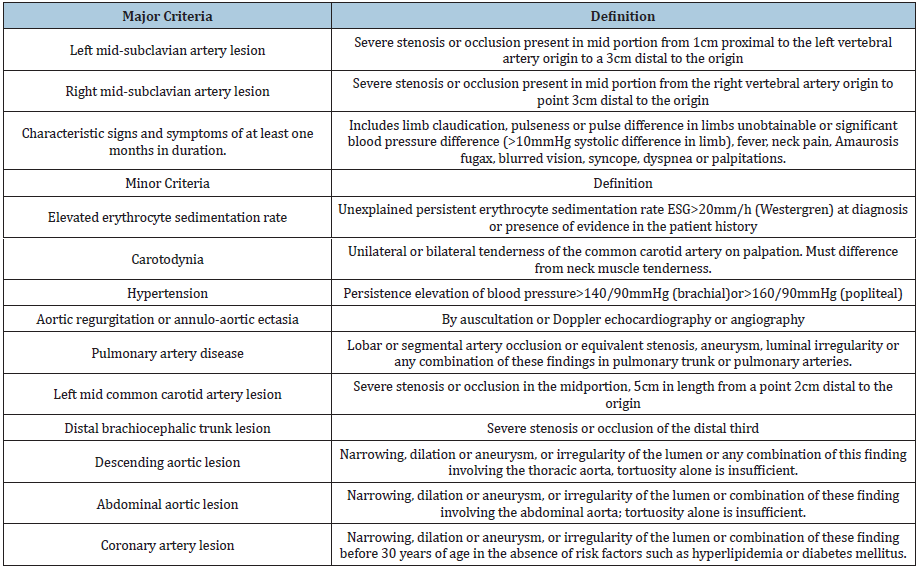
In 1995, Sharma et al. [23] made modifications to the Ishiwaka criteria and created new criteria for the TA diagnosis (Table 2), removed the mandatory criterion for age under 40 years, added new minor criteria such as coronary lesion in patients under 30 years of age without risk factors known. Concluding in three major criteria and ten minor criteria. The diagnosis of Takayasu’s Arteritis according to Sharma would be confirmed with two major criteria or one major and two or four minors. These new criteria have a greater sensitivity of 92.5% and the same specificity 95% when comparing it with American College of Rheumatology criteria [23].
Table 2:Ishikawa criteria for modified takayasu arteritis according to Sharma et al. [23].
Regarding angiographic findings, the Nasu classification was used in the past, in which the involvement of the thoracic and abdominal aorta was not relevant. In 1996 Numano et al. [24] Found that the involvement of the descending and abdominal aorta was more common in South America and Asia with respect to Japan and created a new classification that is currently used which has five types [24]:
1. Type I: located in the supra-aortic branches of the aortic
arch.
2. Type IIa: affects the ascending aorta and the aortic arch
with its branches.
3. Type IIb: involvement of the ascending aorta, aortic arch
with its branches and distal thoracic aorta falling.
4. Type III: includes the descending thoracic aorta,
abdominal and / or renal arteries.
5. Type IV: affects the abdominal aorta and / or the renal
arteries.
6. Type V: combines the findings of type IIb and IV.
Vascular abnormalities in Takayasu’s Arteritis can be studied through magnetic resonance imaging, CT angiography, and ultrasound, with conventional angiography being the “Gold standard”. Each method has advantages and disadvantages and should be used depending on the availability of the method and the type of patient. Conventional angiography is an invasive technique and provides less sensitivity to evaluate the widening and inflammation of the arterial wall, but it continues to be the “gold standard” for specifying and delimiting the different stenosis and occlusions. When aneurysms are present, the preferred study technique is axial tomography. Positron emission tomography promises to be as useful a tool as angiography in detecting the extent and severity of stenosis, but studies are still lacking to establish it as one of the primary tests. Although magnetic resonance imaging does not provide the same value as angiography in terms of the detail of stenosis, it is used in many cases because it is a non-invasive technique that does not involve ionizing radiation, therefore, it is the preferred technique for patient follow-up.
Differential Diagnosis
Certain congenital diseases can affect the extracellular matrix of the aorta and produce aortic insufficiency such as Marfan syndrome, Ehlers Danlos syndrome, but these conditions do not cause stenosis in the great vessels.
Treatment
Medical therapy
Corticosteroids remains the most important active treatment.
Prednisolone from 0.5mg to 1mg/Kg per day is indicated for the
active phase of the disease. The active phase of the disease refers to
the onset or worsening of fever (in the absence of another cause),
increased erythrocyte sedimentation rate, signs or symptoms of
inflammation or vascular ischemia (claudication, absence of pulse)
and typical angiographic lesions. Only 15% of patients do not
present with active disease. The initial dose of prednisolone should
be maintained for 4 to 12 weeks before starting a gradual decrease.
With this management, two thirds of patients present with remission
of the active phase, but more than half of these present relapses. In
the event of relapses, it is recommended to increase the initial dose
of prednisolone or add an immunosuppressive agent. The agents
used have been methotrexate, azatrioprine, cyclosphosphamine,
mycophenolate, and tacrolimus. These cytotoxic agents are usually
continued for a year after remission of symptoms. There is no study
that supports the choice of one of these molecules over another.
Metrotexate is the most used so far, for its safety and easy handling,
with an initial dose of 0.3mg/Kg / week without exceeding 15mg/
week in the initial week, up to its maximum dose of 25mg/week in
achieving remission upto 81% [25] and also decrease the dose of
corticosteroids.
Although there is no global definition of Takayasu’s Arteritis
refractory on management, some studies adopt the proposal of the
Takayasu’s Arteritis study group from Turkey [26], which defines it
as clinical or angiographic progression despite treatment with the
presence of these characteristics:
a. Prednisolone greater than 7.5mg/day and use of
immunosuppressive agents for more than six months.
b. New surgical interventions due to persistence of the
disease
c. More than three exacerbations per year
d. Death associated with active disease.
In recent years, studies of biological agents as a management for refractory Takayasu’s Arteritis have increased. The main biological agents studied have been: Inhibitor of the anti-tumor necrosis factor alpha (Etarnecep, Infliximab) as well as the monoclonal antibody (Tocilizumab), have shown a remission of up to 60% of cases of refractory Takayasu’s Arteritis with the average use of 7 years and has allowed to reduce the dose of corticosteroid [27].
Surgical management
Management of patients with Takayasu’s Arteritis usually
includes steroids during the active phase and treatment of
hypertension during the fibrotic phase. But the complications of
Takayasu’s Arteritis in the chronic phase are usually due to stenosis
or aneurysms of the aorta and great vessels. Uncontrolled studies
have been conducted comparing endovascular revascularization vs
conventional surgery. These studies have proposed that the choice
of revascularization therapy depends on the characteristics of the
lesion, with the percutaneous technique being preferred for short
lesions and difficult-to-access arteries or patients at a high risk
[28]. However, the percentage of restenosis reported with both
stent and balloon is approximately 71.4% at 1.3 years vs 31% at
3 years for Bypass [29,30]. Consequently, conventional surgical
therapy is preferred for stenosis or occlusions in long segments,
however, long-term results for bypass are not as optimal as for
patients without Takayasu arteritis. There are several reasons
that generate high restenosis; generally, they are long lesions, the
vessels are more fibrotic, and there is a persistent state of inflation
in the vessel despite clinical and laboratory improvement [29]. A
report from a small center suggests that the use of a covered stent
may improve restenosis due to the isolation of blood flow to the
vessel walls [30].
20% of patients who present dilated ascending aorta require
aortic valve replacement since the aortic regurgitation generated
by the dilation can lead to left ventricular dysfunction [31].
Stenosis of the mesenteric artery and the celiac artery is usually
asymptomatic, infrequent and in particular cases require surgical
management. Regarding the renal artery stenosis that causes
renovascular hypertension, studies have shown that long-term
balloon angioplasty has similar benefits compared to surgery and
stent angioplasty. Suggesting reserving stent angioplasty for cases
where balloon angioplasty fails and surgery only for patients in
whom angioplasty is not indicated or stent angioplasty has failed
[32,33].
Conclusion
Takayasu’s Arteritis is a disease of somewhat uncertain ethology due to the diversity of factors that can affect it. Its manifestations lie in the vascular condition it produces, with the aorta and its large vessels being the most affected. This vasculitis seems more prevalent in South West Asia and despite having been described for several years in Europe and America, only series of cases have been reported, which could indicate a possible underdiagnosis. Knowledge of this vasculitis is important since its timely management can prevent the progression of the disease and the presentation of vascular complications. The management of a patient with Takayasu’s Arteritis will continue to be a challenge for clinicians, since there are no single standard and management decisions are generally based on the recommendation of experts, so each case must be individualized to offer the most appropriate therapy and reduce the occurrence of complications.
References
- Kerr GS, Hallahan CW, Giordano J, Leavitt RY, Fauci AS, et al. (1994) Takyasu arteritis. Ann Intern Med 120(11): 919-929.
- Numano F, Okawara M, Inomata H, Kobayashi Y (2000) Takayasu’s arteritis. Lancet 356(9234): 1023-1025.
- Caccamise WC, Whitman JF (1952) Puleseless disease: A preliminary case report. Am Heart J 44(4): 629-633.
- Numano F, Kakuta T (1996) Takayasu arteritis five doctors in the history of Takayasu arteritis. Int J Cardiol 54(Suppl): 1-10.
- (1908) Takayasu M: A case of a peculiar change in the central retinal vessels. Acta Soc Ophthalmol Jpn 12: 554.
- Shimizu K, Sano K (1951) Pulseless disease. J Neuropathol Clin 1(1): 37-47.
- Arun R (2015) Analysis of evidence to determine the link between Takayasu’s arteritis and Tuberculosis. Indian Journal of Rheumatology 10(1): 2-9.
- Arnaud L (2011) Pathogenesis of Takayasu's arteritis: A 2011 update. Autoimmunity Reviews 11(1): 61-67.
- Arnaud L (2006) Takayasu’s arteritis: An update on physiopathology. Eu J Int Med 17(4): 241-246.
- Sharma BK, Sagar S, Chugh KS, Sakhuja V, Rajachandran A, et al. (1985) Spectrum of renovascular hypertension in the young in North India: A hospital-based study on occurrence arid clinical features. Angiology 36(6): 370-378.
- Maxwell MH, Bleifer KH, Frauklin SS, Varady PD (1972) Cooperative study of renovascular hypertension: Demographic analysis of the study. J Am Med Assoc 220(9): 1195-1204.
- Alibaz-Oner F, Direskeneli H (2015) Update on Takayasu's arteritis. Presse Med 44: e259-e265.
- Sharma S (1998) A possible role of sex in determining distribution of lesions in Takayasu Arteritis. Int J Cardiol 66( Suppl 1): S81-S84.
- Cañas CA, Jimenez CA, Ramirez LA, Uribe O, Tobón I, et al. (1998) Takayasu arteritis in Colombia. Int J Cardiol 66 (Suppl 1): S73-S79.
- Dufrechou CA, Cedrés SA (2006) Arteritis de Takayasu. Rev Med Urug 22: 236-240.
- Dabague J, Reyes Pedro A (1996) Takayasu arteritis in Mexico: A 38-year clinical perspective through literature review. Int J Cardiol 54 (Suppl): 103-109.
- Buzaid AC, Milani JR, Calich Y, Pereira VG (1985) Arterite de Takayasu: estudo de 16 casos, aspectos clinicos, laboratoriais, e revis, oda literatura. Rev Assoc Md Bras 3(5-6): 85-90.
- López M, Gonzalez P, Esther N (1987) Takayasu’s arteritis in Puerto rico: A clinical study. Bol Asoc Md PR 79: 230-235.
- Ishikawa K (1988) Diagnostic approach and proposed criteria for the clinical diagnosis of Takayasu's arteriopathy. J Am Coli Cardiol 12(4): 964-972.
- Sharma B (1996) Diagnostic criteria for Takayasu arteritis. International Journal of Cardiology 54 (Suppl) : S127-S133.
- Maksimowicz K, Gary SH (2007) Takayasu arteritis: What is the long-term prognosis? Rheum Dis Clin N Am 33(4): 777-786.
- Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, et al. (1990) The American college of rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum 33(8):1129-1134.
- Sharma BK, Iliskovic NS, Singal PK (1995) Takayasu arteritis may be underdiagnosed in North America. Can J Cardiol 11(4): 311-316.
- Hata A, Noda M, Moriwaki R, Numano F (1996) Angiographic findings of Takayasu arteritis: New classification. Int J Cardiol 54(Suppl): 155-163.
- Hoffman GS, Leavitt RY, Kerr GS, Rottem M, Sneller MC, et al. (1994) Treatment of glucocorticoid resistant or relapsing Takayasu arteritis with methotrexate. Arthritis Rheum 37(4): 578-582.
- Saruhan-Direskeneli G, Travis H, Kenan A, Gokhan K, Patrick C, et al. (2013) Identification of multiple genetic susceptibility loci in Takayasu arteritis. Am J Hum Genet 93(2): 298e-305e.
- Hoffman GS, Merkel PA, Richard D, Deborah J, Patrick L (2004) Anti-tumour necrosis factor therapy in patients with difficult to treat Takayasu arteritis. Arthritis Rheum 50(7): 2296-2304.
- Sharma S, Gupta H, Saxena A, Kothari SS, Taneja K, et al. (1998) Results of renal angioplasty in nonspecific aortoarteritis (Takayasu disease). J Vasc Intev Radiol 9(3): 429-435.
- Keser G, Direskeneli H, Aksu K (2011) Management of Takayasu arteritis: a systematic review. Rheumatology 53(5):793-801.
- Qureshi MA, Martin Z, Greenberg RK (2011) Endovascular management of patients with Takayasu arteritis: stents versus stent grafts. Semin Vasc Surg 24(1): 44-52.
- Zhang Y, Fan P, Zhang H, Wenjun M, Lei Song, et al. (2019) Surgical treatment in patients with aortic regurgitation due to Takayasu arteritis. Ann Thorac Surg 110(1): 165-171.
- Kinio H, Kafa AT (2015) The results of treatment in renal artery stenosis due to Takayasu disease: Comparison between surgery, angioplasty, and stenting. A monocentrique retrospective study. Giornale di Cirugía 36(4): 161-167.
- Ambrish S, Debashish D, Salman H, Abul KN, Ashish M, et al. (2020) Efficacy and safety of tocilizumab in treatment of Takayasu arteritis: A systematic review of randomized controlled trials. Modern Rheumatology 31(1): 197-204.
© 2021 Paul Gamboa. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






