- Submissions

Full Text
COJ Reviews & Research
A Brief Review of Secondary Plant Metabolites as Anticancer Agents
Naicker L and Mohanlall V*
Department of Biotechnology and Food Technology, South Africa
*Corresponding author: Mohanlall V, Department of Biotechnology and Food Technology, Faculty of Applied Sciences, Durban University of Technology, South Africa
Submission: April 09, 2020; Published: May 18, 2020

ISSN 2639-0590Volum2 Issue4
Abstract
Plants have provided a source of medicine from the beginning of human history and are the core of modern medicine. Moreover, plant-based drug discovery has led to the development of various anticancer drugs (such as vincristine, vinblastine, etoposide, paclitaxel, camptothecin, topotecan and irinotecan). The use of botanical, photochemical, biological and molecular techniques have facilitated the discovery of novel secondary metabolites from native and indigenous plants that can inhibit the human topoisomerase II enzyme (target for anticancer drugs) and kill cancer cells. Therefore, the aim of this review was to further investigate the anticancer activity of secondary metabolites from native and indigenous plants and determine the classes of compounds that contributed towards its activity.
Keywords: Anticancer; Secondary metabolites; Topotecan; Etoposide; Vincristine
Introduction
Thousands of years ago, ancient civilizations in many countries such as China, India and Thailand used plants for medicinal purposes [1-3]. The traditional use of plants has continued into modern times as the World Health Organization (WHO) estimated that 80% of people living in developing countries depend on traditional medicine (mostly herbal medicines) to fulfill their primary health care needs [4]. Moreover, herbal medicines are highly valuable in the international marketplace. In Western Europe, herbal medicine revenue amounted to US $5 billion in 2003-2004. In China, sales of herbal medicine products reached US $14 billion in 2005. In Brazil, herbal medicine revenue reached US $160 million in 2007 [4]. Locally, over 70 plant species are used in Africa to treat both veterinary and human patients [5]. In addition, South Africa has a long history of using plants in traditional healing as it hosts a variety of around 30,000 flowering species [6].
This accounts for almost 10 % of the world’s higher plant species [7]. Thus, plants have been an integral component of the health care system both locally and internationally. There are several advantages to using plants for treat cancer. The complex synergistic interaction of various compounds in plants allows for the designing of herbal formulations that attack cancerous cells without harming normal cells of the body. This is possible as some plants can protect the body against cancer by enhancement of its detoxification functions. Certain plants contain biological response modifiers that can prevent the growth of cancer by modulating the activity of specific hormones and enzymes. Some plants have good immunomodulatory and antioxidant properties which can promote anticancer activity [8,9].
Moreover, a survey documented that over 60% of cancer patients depend on the use of vitamins or herbs as therapy [10]. Therefore, medicinal plants play an important role in the treatment of cancer. Although traditional practices involve the use of complex plant extracts, scientists have focused on purifying and identifying Independently Active Compounds (IACs) from plant extracts. The advantage is that once the structures of the IACs are determined they can be chemically or semi- chemically synthesized. The disadvantage is that some compounds found in plants act in a synergistic manner with other compounds to bring about biological activity.
Plants produce two distinct groups of compounds. The first group consists of the primary metabolites which include sugars (constituents of structural and nutritional elements), amino acids (constituents of structural elements and enzymes), lipids (constituents of membranes and nutritional elements) and nucleotides (constituents of genes). These metabolites are needed for the growth of the plant [11]. The second group consists of the secondary metabolites which include five types of compounds: polyketides, isoprenoids (terpenoids), alkaloids, phenylpropanoids and flavonoids (polyphenols) [12]. They are responsible for metabolic and/or growth regulation, lignification, the colouring of plant parts and protecting the plant against pathogen attack. Secondary metabolites are important constituents as they possess pharmaceutical properties [11,13]. Thus, these compounds are responsible for any possible anticancer activity.
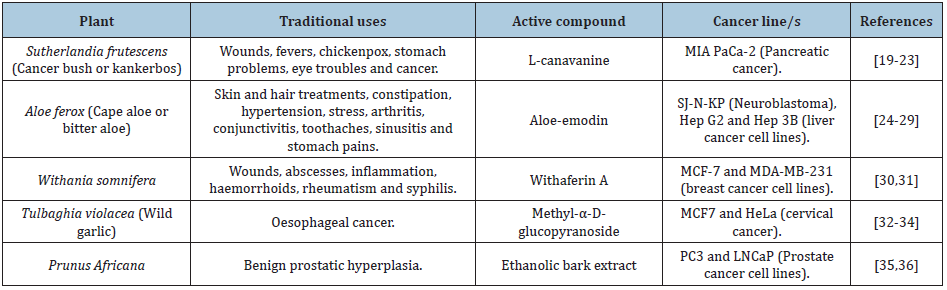
Scientists have discovered many anticancer compounds from plants. A classic example is the discovery of a diterpene known as taxol which was isolated from the Pacific yew tree, Taxus baccata [13]. This research began in 1958 when the National Cancer Institute (NCI) (United States of America) screened 35, 000 plants for anticancer activity. Fortunately, a breakthrough was made in 1963 when Drs M. Wall and M.C. Wani of the Research Triangle Institute (North Carolina) found that the extract from the bark of T. baccata exhibited anticancer activity [14]. Twenty years later human clinical studies began and taxol was shown to be active against ovarian cancer which was incurable during the 80’s. In order to further develop taxol, the NCI issued a contract to Bristol Myers- Squibb in the United States [15]. Nowadays Taxol is semi-chemically synthesized as paclitaxel by using the needles and twigs from yew species which are cultivated under agricultural conditions. This drug has been approved by the Food and Drug Administration (FDA) for treating ovarian, breast, lung and other types of cancers. It is sold under the trade name Taxol [13]. Table 1 shows other plant-derived anticancer compounds that have been commercialized. South Africa has a variety of anticancer plants (Table 2) [13-36].
Table 1: Plant-derived anticancer compounds that have been commercialized.
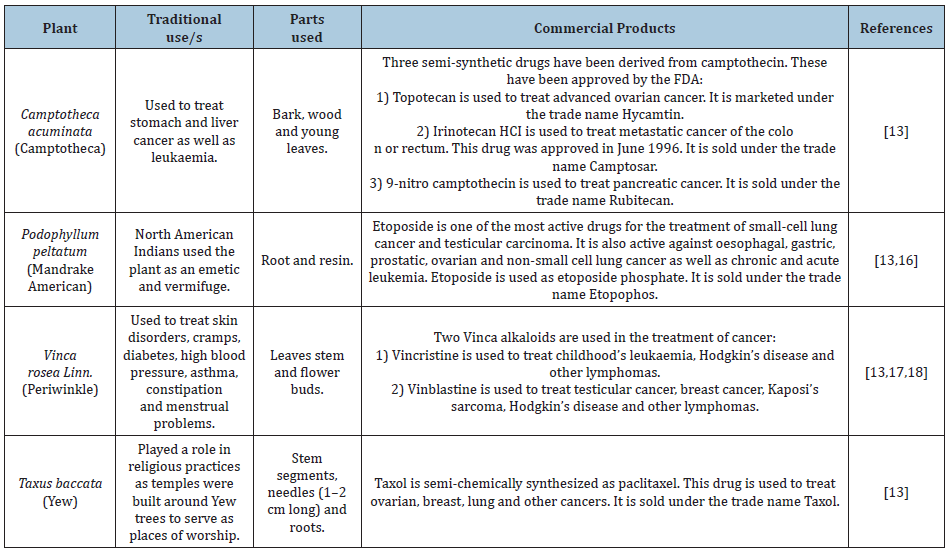
Table 2: South African plants that have shown to exhibit anticancer activity.
Treatment of cancer
The treatment of cancer can involve surgical intervention, radiation, chemotherapy, targeted therapy and/or other therapies [37]. The objective of any cancer treatment is to remove all cancerous tissue without harming normal tissues. Severe cases of cancer are treated by surgery which allows for the removal of cancerous tissue with less damage to healthy tissues but surgery can also have certain risks (such as bleeding, drug reactions and damage to nearby tissues or organs) and side effects (such as pain and infection) [38,39]. In less severe cases of cancer, radiation or chemotherapy can be used to remove the cancerous tissue but these treatments are limited due to the toxicity imposed on healthy tissues [40].
The side effects of radiation therapy depend on the part of the body that is exposed to the treatment. The following side effects may be experienced: diarrhoea, hair loss, fatigue, mouth and throat changes, nausea, vomiting, sexual changes, fertility changes, skin changes and urinary changes [41]. The side effects of chemotherapeutic drugs may include: anemia, appetite changes, bleeding problems, constipation, diarrhoea, fatigue, hair loss, infection, memory changes, mouth changes, throat changes, nausea, vomiting, nerve changes, pain, sexual changes, fertility changes, skin changes, nail changes, swelling and urination changes [42]. In addition, a major complication that can occur while chemotherapy is administered is multi-drug resistance (MDR). This occurs as a result of
- The up-regulation of membrane proteins which pump the drugs out of the cell to decrease the intracellular drug concentration,
- The interruption of the balance between bioactivation and detoxification pathways,
- Alterations in DNA repair response and
- Alterations in apoptosis response. Thus, MDR can hinder the success of treating cancers by chemotherapy [43].
On the other hand, some cancers are treated with targeted therapy. This involves the use of monoclonal antibody drugs which target and disrupt molecular pathways that are responsible for promoting tumorigenesis [44]. However, some of these drugs are associated with serious side effects. For example, trastuzumab causes cardiotoxicity in women with metastatic breast cancer [45]. Cetuximab and panitumumab are used for the treatment of refractory metastatic colorectal cancer but these drugs cause a skin rash on the face and upper torso [46]. Rituximab is used to treat non-Hodgkin’s lymphoma but it is associated with serious infections such as the John Cunningham virus which infects the central nervous system [47,48]. Hence due to the side effects caused by the current cancer treatments, there is a need to discover and develop new drugs to effectively and safely treat cancer.
How do anticancer drugs induce apoptosis?
Anticancer drugs are classified according to their mechanism of action. There are four main classes of anticancer drugs: antimetabolites, genotoxic agents, anti-mitotic agents and targeted therapies [49,50] (Table 1). Through their mechanism of action, anticancer drugs can initiate apoptosis by switching on the expression of signal molecules such as Fas and FasL (for initiation of the extrinsic pathway type I and II) or p53 (for initiation of the intrinsic pathway) [51]. For instance, emodin induces topoisomerase II inhibition which causes DNA damage that activates the expression of FasL for initiation of the extrinsic pathway type I [52]. On the other hand, 6-Mercaptopurine incorporates itself into DNA and subsequently activates the expression of p53 for initiation of the intrinsic pathway [53]. Thus, anticancer drugs work in a very intricate manner to facilitate the death of cancer cells (Table 3) [54-60].
Table 3:

Conclusion
Anticancer treatment and therapy have evolved significantly since the 1820s when Dr James Arnott introduced cryotherapy as a mechanism to freeze tumors in breast and uterine cancer patients. Radiotherapy and immunotherapy were the main pillars of treatment for cancers in the late 19th century until the introduction of the chemotherapeutic drug, Mustine in 1942. The introduction of plant metabolites has contributed to the improvements in outcomes related to the treatment of cancer. Advances in phytochemical characterization and understanding the pharmacokinetic properties of plant metabolites are proving effective to increase the survival rate of cancer patients. New discoveries in the identification of novel metabolites from plants and novel targets in cancer cells are of paramount importance to our successful treatment of cancer.
Acknowledgement
The authors wish to thank the Durban University of Technology and the Department of Higher Education and Training (DoHET) for providing facilities and the National Research Foundation (NRF) for funding.
References
- Chang HM, But PPH (1986) Pharmacology and applications of Chinese materia medica. World Scientific Publishing, Singapore.
- Kapoor LD (1990) Handbook of Ayurvedic Medicinal Plants, CRC Press, Florida, USA.
- Subchareon P (1998) Handbook of anticancer: Thai traditional medicine: New concept for treated cancer, Bangkok.
- Chaudhary SA, Gadhvi KV, Chaudhary AB (2010) Comprehensive review on world herb trade and most utilized medicinal plant. International Journal of Applied Biology and Pharmaceutical Technology 1: 510-517.
- Stark TD, Mtui DJ, Balemba OB (2013) Ethno pharmacological survey of plants used in the traditional treatment of gastrointestinal pain, inflammation and diarrhea in Africa: Future perspectives for integration into modern medicine. Animals 3(1): 158-227.
- Louw CAM, Regnier TJC, Korsten L (2002) Medicinal bulbous plants of South Africa and their traditional relevance in the control of infectious diseases. Journal of Ethno pharmacology 82: 147-154.
- Van WYK BE, Gericke N (2000) People’s plants: A guide to useful plants of Southern Africa, South Africa, Briza Publications, South Africa.
- Larkin T (1983) Herbs are often more toxic than magical. FDA Consumer, 17: 4-11.
- Saxe TG (1987) Toxicity of medicinal herbal preparations. Am Fam Physician 35(5): 135-142.
- Sivalokanathan S, Ilayaraja M, Balasubramanium MP (2005) Efficacy of Terminalia arjuna (Roxb.) on N-nitrosodiethylamine induced hepatocellular carcinoma in rats. Indian Journal of Experimental Biology 43(3): 264-267.
- Payne G, Bringi V, Prince C, Shuler M (1991) Plant cell and tissue culture in liquid systems, Munich, Hanser Publishers, Germany.
- Verpoorte R (2000) Metabolic Engineering of Plant Secondary Metabolism, Kluwer Academic Publishers, Germany.
- Kintzios SE, Barberaki MG (2004) Plants that Fight Cancer, CRC Press, USA.
- Cragg GM (1998) Paclitaxel (Taxol): A success story with valuable lessons for Natural Product Drug discovery and development. Medicinal Research Reviews 18(5): 315-331.
- Rowinsky EK, Cazenave LA, Donehower RC (1990) Taxol: A novel investigational anti-microtubule agent. J Natl Cancer Inst 82(15): 1247-1259.
- Schacter L (1996) Etoposide phosphate: what, why, where, and how? Semin Oncol 23, 1-7.
- Canellos GP1, Anderson JR, Propert KJ, Nissen N, Cooper MR, et al. (1992) Chemotherapy of advanced hodgkin’s disease with MOPP, BVD, or MOPP alternating with ABVD. N Engl J Med 327(21): 1478-1484.
- Samuelsson G (1992) Drugs of Natural Origin: A textbook of Pharmacognosy, Swedish Pharmaceutical Press,
- Crooks PA, Rosenthal GA (1996) Use of L-canavanine as a chemotherapeutic agent for the treatment of pancreatic cancer, USA.
- Swaffar DS, Ang CY, Desai PB, Rosenthal GA (1994) Inhibition of the growth of human pancreatic cancer cells by the arginine antimetabolite L-canavanine. Cancer Res 54(23): 6045-6048.
- Thomson S (2002) Canavanine toxicity: Is Sutherlandia a healthy herb or potential poison? HIV Positive and AIDS Sufferers Beware: The Remedy may be Worse than the Alleged Disease. Gaia Research Institute, USA.
- Van WYK BE (1997) Medicinal Plants of South Africa, Briza publishers, South Africa.
- Xaba PMA, Notten A (2003) Sutherlandia frutescens (L.) R.Br. Kirstenbosch: South African National Biodiversity Institute, South Africa.
- Crouch N, Symmonds R, Spring W, Diederichs N (2006) Facts sheets for growing popular medicinal plant species. In: Diederichs N (Ed.), Commercializing medicinal plants-A Southern African Guide. Sun Press, South Africa.
- Kuo PL, LIN TC, Lin CC (2002) The anti-proliferative activity of aloe-emodin is through p53-dependent and p21-dependent apoptotic pathway in human hepatoma cell lines. Life Sci 71(16): 1879-1892.
- Pecere T, Gazzola MV, Mucignat C, Parolin C, Vecchia FD, et al. (2000) Aloe-emodin is a new type of anticancer agent with selective activity against neuro ectodermal tumors. Cancer Res 60(11): 2800-2804.
- Pujol J (1990) Natura Africa-Herbalist Handbook, Natural healers foundation, Durban, South Africa.
- Street RA, Prinsloo G (2013) Commercially important medicinal plants of South Africa: A review. Journal of Chemistry 1-16.
- Watt JM, Breyer BMG (1962) The medicinal and poisonous plants of Southern and Eastern Africa. Livingstone, London, UK.
- Stan SD, Hahm ER, Warin R, Singh SV (2008) Withaferin A causes FOXO3a- and Bim-dependent apoptosis and inhibits growth of human breast cancer cells in vivo. Cancer Res 68(18): 7661-7669.
- Welman M (2011) Withania somnifera (L.) Dunal. Pretoria: South African National Biodiversity Institute, South Africa.
- Bungu L, Frost CL, Brauns SC, Van De Venter M (2006) Tulbaghia violacea inhibits growth and induces apoptosis in cancer cells in vitro. African Journal of Biotechnology 5(20): 1936-1943.
- Lyantagaye SL (2013) Methyl-α-D-glucopyranoside from Tulbaghia violacea extract induces apoptosis in vitro in cancer cells. Bangladesh Journal of Pharmacology 8(2): 93-101.
- Van WYK BE (1997) Medicinal Plants of South Africa, Briza publishers, South Africa.
- Edgar AD, Levin R, Constantinou CE, Denis L (2007) A critical review of the pharmacology of the plant extract of Pygeum africanum in the treatment of LUTS. Neurourol Urodyn 26(4): 458-463.
- Vinceti B, Loo J, Gaisberger H, van Zonneveld MJ, Schueler S, et al. (2013) Conservation priorities for Prunus africana defined with the aid of spatial analysis of genetic data and climatic variables. PLoS One 8(3): e59987.
- National Cancer Institute (2015) Types of Treatment, USA.
- American cancer association (2014) What are the risks and side effects of cancer surgery? USA.
- Erhabor O, Adias TC (2011) From whole blood to component therapy: The economic, supply/demand need for implementation of component therapy in sub-Saharan Africa. Transfus Clin Biol 18(5-6): 516-526.
- Brannon PL, Blanchette JO (2004) Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev 56(11): 1649-1659.
- National Cancer Intstitute (2014) Radiation Therapy Side Effects Series, USA.
- National Cancer Intstitute (2014) Chemotherapy Side Effects Series, USA.
- David SR, Chunja Lee, Shairoz R, Edwin CC, Rachel LC, et al. (2005) Cancer chemotherapy and drug metabolism. Drug Metabolism and Disposition 33(8): 1083-1096.
- Majidi J, Barar J, Baradaran B, Abdolalizadeh J, Omidi Y, et al. (2009) Target therapy of cancer: Implementation of monoclonal antibodies and nanobodies. Hum Antibodies 18(3): 81-100.
- Force T, Krause DS, Van Etten RA (2007) Molecular mechanisms of cardio toxicity of tyrosine kinase inhibition. Nat Rev Cancer 7(5): 332-344.
- Jean GW, Shah SR (2008) Epidermal growth factor receptor monoclonal antibodies for the treatment of metastatic colorectal cancer. Pharmacotherapy 28(6): 742-754.
- Aksoy S, Harputluoglu H, Kilickap S, Dede DS, Dizdar O, et al. (2007) Rituximab-related viral infections in lymphoma patients. Leuk Lymphoma 48(7): 1307-1312.
- Carson KR, Focosi D, Major EO, Petrini M, Richey EA, et al. (2009) Monoclonal antibody-associated progressive multifocal leukoencephalopathy in patients treated with rituximab, natalizumab, and efalizumab: A Review from the Research on Adverse Drug Events and Reports (RADAR) Project. Lancet Oncol 10(8): 816-824.
- Druker BJ (2003) David A Karnofsky Award lecture. Imatinib as a paradigm of targeted therapies. J Clin Oncol 21: 239s-245s.
- Gascoigne KE, Taylor SS (2009) How do anti-mitotic drugs kill cancer cells? Journal of Cell Science 122: 2579-2585.
- Igney FH, Krammer PH (2002) Death and anti-death: Tumour resistance to apoptosis. Nature Reviews Cancer 2(4): 277-288.
- Li Y, Luan Y, Qi X, Li M, Gong L, et al. (2010) Emodin triggers DNA double-strand breaks by stabilizing topoisomerase II-DNA cleavage complexes and by inhibiting ATP hydrolysis of topoisomerase II. Toxicol Sci 118(2): 435-443.
- Payne S, Miles D (2008) Mechanisms of anticancer drugs. In: John C Watkinson, Ray W Clarke (Eds.), Scott-Brown's Otorhinolaryngology: Head and neck surgery (7th edn), CRC Press, USA.
- Adam de Beaumais T, Fakhoury M, Medard Y, Azougagh S, Zhang D, et al. (2011) Determinants of mercaptopurine toxicity in paediatric acute lymphoblastic leukemia maintenance therapy. Br J Clin Pharmacol 71(4): 575-584.
- Mantadakis E, Cole PD, Kamen BA (2005) High-dose methotrexate in acute lymphoblastic leukemia: where is the evidence for its continued use? Pharmacotherapy 25(5): 748-755.
- Champoux JJ (2001) DNA topoisomerases: Structure, function, and mechanism. Annual Review of Biochemistry 70: 369-413.
- Smith L1, Watson MB, O'Kane SL, Drew PJ, Lind MJ, et al. (2006) The analysis of doxorubicin resistance in human breast cancer cells using antibody microarrays. Molecular Cancer Therapeutics 5(8): 2115-2120.
- Vermorken JB, Harper PG, Buyse M (1999) The role of anthracyclines in epithelial ovarian cancer. Annals of Oncology 10: 43-50.
- Smith BD (2011) Imatinib for chronic myeloid leukemia: The impact of its effectiveness and long-term side effects. Journal of the National Cancer Institute 103(7): 527-529.
- Bilal P, Yi He Ling, Leonard L, Franco M, Roman S, et al. (2011) Bortezomib: Understanding the mechanism of action. Albert Einstein College of Medicine, USA.
© 2020 Mohanlall V. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






