- Submissions

Full Text
COJ Reviews & Research
Evaluation of Hair Growth after Exposure with Biofield Energy Treated Williams Medium E Using Mouse Vibrissae Hair Follicle Organ Culture
Alice Branton1 and Snehasis Jana2*
1Trivedi Global, Inc., USA
2Trivedi Science Research Laboratory Pvt. Ltd, India
*Corresponding author: Snehasis Jana, Trivedi Science Research Laboratory Pvt. Ltd, Bhopal, Madhya Pradesh, India
Submission: December 11, 2018;Published: January 04, 2019

ISSN: 2639-0590 Volume2 Issue1
Abstract
Hair plays a lucrative outlook on the human body and also contribute an exciting part in social and sexual communication. Loss of hair follicle would potentially lead to various skin disorders. For this consequence, the present study was investigated for the potential of the Biofield Energy Healing (The Trivedi Effect®) Treated test item (William’s Medium E) on the vibrissae hair follicle organ culture cells for the assessment of hair cell growth and development in vitro. The test item was divided into two parts. One part was defined as the untreated, where no Biofield Energy Treatment provided, while the other part was defined as the Biofield Energy Treated test item, which received the Biofield Energy Healing Treatment by a renowned Biofield Energy Healer Alice Branton. The study parameters like bulb thickness and formation of telogen were assessed using cell-based assay with the help of UTHSCSA Image tool version 3. The experimental results showed that the untreated test item group showed 20% and 26.67% increased bulb thickness on day 5 and 7, respectively compared to day 1. Besides, the percent telogen follicle was found as 86%, 86%, and 100% on day 3, 5, and 7, respectively of the Biofield Energy Treated test item group. The overall results demonstrated that the Biofield Energy Treatment has the potential for hair growth promotion as evident via an increased formation of telogen. Therefore, the Biofield Energy Healing (The Trivedi Effect®) Treatment might be useful as a hair growth promoter for various treatment of skin injuries and skin-related disorders like necrotizing fasciitis, actinic keratosis, sebaceous cysts, diaper rash, decubitus ulcer etc.
Keywords: Hair growth; Biofield energy healing; Consciousness energy healing treatment; The Trivedi effect®, Vibrissae hair follicle cells; Bulb thickness
Abbreviations: CAM: Complementary and Alternative Medicine; PBS: Phosphate-Buffered Saline; DPCs: Dermal Papilla Cells; UT-TI: Untreated Test Item
Introduction
Lots of assays are routinely used to assess hair growth, while hair follicle organ culture model is one of the most popular and powerful in vitro systems [1]. Rodent vibrissa follicles are regular, predictable, and relatively short growth cycles. Hence, considering these properties authors choose mouse vibrissa follicles as a test model for the assessment of hair cycle and compared morphologic changes in culture [2]. With the measurement of follicular activity concerning bulb thickness and improvement of anagen initiation, regression of catagen, and finally shifting of hair bulb i.e., telogen formation is the main criteria for hair growth [3]. The hair follicle is consist of mainly two components, one is epithelial components and the others are dermal components. Hair growth is regulated by the division of the hair follicle matrix cells under control of the dermal papilla. Three different stages of hair growth can be identified, an active phase (anagen) during which hair growth occurs, an intermediate regressive (catagen) stage and a resting phase (telogen) during which no cell proliferation occurs [4]. The positive control used in this experiment i.e., minoxidil because from literature reported that it can directly promote hair growth via the stimulation of growth factor release from adipose-derived stem cells dermal papilla and epithelial cells [5]. In recent years, several scientific reports and clinical trials have revealed the beneficial effects of Biofield Energy Treatments, which have shown to enhance immune function in cases of cervical cancer patients via therapeutic touch [6], massage therapy [7], etc. Complementary and Alternative Medicine (CAM) therapies are now rising as preferred models of treatment, among which Biofield Therapy (or Healing Modalities) is one approach that has been reported to have several benefits to enhance physical, mental and emotional human wellness. However, as per the data of 2012 from the National Health Interview Survey (NHIS), which indicated that the highest percentage (17.7%) of the Americans used dietary supplements as a complementary health approach as compared with other practices in past years. The National Center of Complementary and Integrative Health (NCCIH) has recognized and accepted Biofield Energy Healing as a CAM health care approach in addition to other therapies, medicines and practices such as natural products, deep breathing, yoga, Tai Chi, chiropractic/osteopathic manipulation, Qi Gong, meditation, massage, special diets, homeopathy, progressive relaxation, rolfing structural integration, guided imagery, acupuncture, relaxation techniques, acupressure, healing touch, essential oils, hypnotherapy, traditional Chinese herbs and medicines, pilates, movement therapy, Reiki, mindfulness, Ayurvedic medicine, aromatherapy, naturopathy, and cranial sacral therapy. Human Biofield Energy has subtle energy that can work effectively [8]. CAM therapies have been practiced worldwide with reported clinical benefits in different health disease profiles [9]. This energy can be harnessed and transmitted by the experts into living and non-living things via the process of Biofield Energy Healing. Biofield Energy Treatment (The Trivedi Effect®) has been published in numerous peer-reviewed science journals with significant outcomes in many scientific fields such as cancer research [10,11], microbiology [12-15], biotechnology [16,17], pharmaceutical science [18- 21], agricultural science [22-25], materials science [26-29], nutraceuticals [30,31], skin health, human health and wellness.
Based on the literature information and importance of Biofield Energy Healing Treatment on various fields, the authors sought to evaluate the impact of the Biofield Energy Treatment (The Trivedi Effect®) on the test item (William’s Medium E) for hair cells growth activity with respect to the assessment of different hair growth parameters like bulb thickness and telogen formation using standard assays in vibrissae hair follicle organ culture cells with the help of UTHSCSA Image tool version 3.
Materials and Methods
Chemicals and reagents
William’s Medium E (phenol-free) with growth factors, antibiotics solution (penicillin-streptomycin), and DMEM (phenolred free) were procured from HiMedia, India. Minoxidil sulphate (positive control) was purchased from Clearsynth Labs Ltd., Mumbai. L-glutamine and fungisone were procured from Gibco, India. Insulin from bovine pancreas, hydrocortisone, vitamin B12, and glucose were obtained from Sigma Chemical Co. (St. Louis, MO). All the other chemicals used in this experiment were analytical grade procured from India.
Isolation and maintenance of vibrissa hair follicles from mice
Vibrissa hair follicles were isolated from 16 days old C57BL/6 mice by microdissection using a standard method with few modification [32]. Briefly, both the left and right whisker pads of C57BL/6 mice were excised out and placed in a 1:1 solution of Earle’s balanced salts solution and phosphate-buffered saline (PBS) supplemented with 100U penicillin per mL and 100mg streptomycin per mL. After that, individual anagen follicles were isolated from the whisker pad and were randomized into different groups and transferred on to a 5cm plastic petri dish containing Earle’s balanced salts solution/PBS (1:1) using one dish per animal. Isolated anagen follicles were maintained in a 24-well plate in William’s medium E (supplemented with growth factors) for 7 days and maintained at 37 °C at 5 % CO2 [33]. William’s Medium E (phenol-free) with growth factors was used as a test system in the present study. Vibrissae hair follicle culture was maintained under William’s Medium E growth medium for routine culture supplemented with 10% FBS [34].
Experimental design
Isolated anagen follicles were grouped into following treatment groups. Group 1 was served as untreated test item (William’s Medium E cells phenol-free supplemented with growth factors). Group 2 was defined as Biofield Energy Treated William’s Medium E. Group 3 was denoted as the positive control, minoxidil sulphate (1mM).
Biofield energy healing approach
The William’s Medium E has used a test item in this experiment. The test item was divided into two parts. One part was considered as the untreated test item, where no Biofield Energy Healing Treatment was provided. Further, the untreated test items group was treated with “sham” healer for comparison purpose. The “sham” healer did not aware about the Biofield Energy Healing Treatment. The second part of the test item was received Biofield Energy Healing Treatment (known as The Trivedi Effect®) under laboratory conditions for 3 minutes through the Alice Branton’s unique Biofield Energy Transmission process to the test item. Biofield Energy Healer in this study did not visit the laboratory, nor had any contact with the test samples. After that, the Biofield Energy Treated and untreated test items were kept in similar sealed conditions and used for the study as per the study plan.
Morphological analysis of vibrissa hair follicles
All the follicles in the well plate were observed daily through the microscope for any morphological changes. Photographs of the individual vibrissae follicles were captured during the course of the study upto day 7. After the completion of the experiment, all the follicles treated with test items and positive control were measured for hair bulb thickness and compared to the respective baseline thickness of day 1 using UTHSCSA Image tool version 3.
Statistical analysis
Data were expressed as the mean ± standard error of the mean (SEM). For statistical analysis, Sigma-Plot (version 11.0) was used as a statistical tool. For two group’s comparison, Student’s t-test was used. Statistically significant values were set at the level of p≤0.05.
Result and Discussion
Assessment of vibrissa hair follicles
The hair follicles cycle occurs through a consecutive sequence of major stages known as the stage of growing (anagen), stage of involuting (catagen), stage of resting (telogen), and finally the stage of shedding (exogen) (phase) [35,36]. This cycle of hair growth is influenced by dermal papilla cells (DPCs); while if the DPCs are in a pathological state that leads to various hair loss related disorders [37-39]. The first true anagen phase mainly develops in the fourth and fifth week of postnatal life. While, the first hair shafts can visible after birth during their final stages of morphogenesis [40]. The reference standard used in this experiment, minoxidil is a wellestablished therapeutic for various types of hair growth-related disorders like alopecia [41]. The vibrissae hair follicle organ culture cells were treated with positive control and untreated test item (William’s Medium E). The percent increased of bulb thickness of both minoxidil sulphate and untreated test item groups are shown in (Figure 1). The study data showed that the bulb thickness in the positive control (minoxidil) group was 1.9±0.29, 2.6±0.37, and 3.3±0.36mm on day 1, 5, and 7, respectively. Additionally, the untreated test item group showed 1.5±0.45, 1.8±0.57, and 1.9±0.60 mm of bulb thickness on day 1, 5, and 7, respectively. Follicles treated with the positive control, minoxidil sulphate showed an increased in hair bulb thickness in follicles by 34.5% and 73.2% on day 5 and day 7, respectively compared to day 1. Moreover, the untreated test item group, follicle was maintained their integrity to some extent with slight increase in hair bulb thickness by 20.9% and 28.2% on day 5 and 7, respectively with respect to day 1 (Figure 1). Follicles were observed to have catagen-like changes with increased in hair bulb thickness in both minoxidil and untreated test item groups.
Figure 1:Assessment of hair follicle growth and development in William’s Medium E in terms of bulb thickness (mm) on vibrissae hair follicle organ culture cells of positive control and untreated test item groups. UT-TI: Untreated test item (William’s Medium E). Values are expressed as Mean ± SEM. *p≤0.05 vs. day 1.
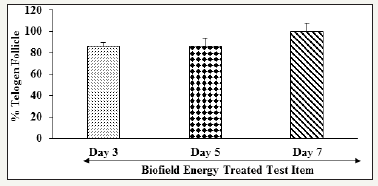
Figure 2:Effect of the Biofield Energy Healing Treatment on vibrissae hair follicle organ culture cells for the assessment of hair follicle growth and development in William’s Medium E in terms of telogen follicles of Biofield Energy Treated test item (William’s Medium E).
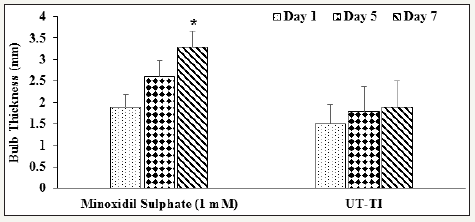
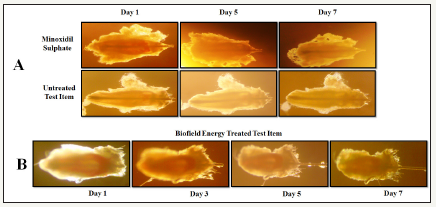
Figure 3:Representative photomicrograph of hair follicle development (anagen - catagen - telogen) of different treatment groups.
A: Initiation of anagen follicle (thick hair bulb);
B: Transformation of initiation, regression of hair bulb, and shifting of the hair shaft (telogen follicle) in the Biofield Energy Treated test item (William’s Medium E) group.
Besides, the Consciousness Energy Treated test item (William’s Medium E) on vibrissae hair follicle organ culture cells and the percent of telogen follicles are presented in (Figure 2). The percentage of telogen follicle was remarkably elevated by 86%, 86%, and 100% on day 3, 5, and 7, respectively in the Biofield Energy Treated test item group compared to day 1 (Figure 2). On day 7, shifting of the hair shaft from its original place was observed in seven out of seven follicles i.e., 100%, which is a hallmark of telogen transition shown in (Figure 3). However, Biofield Energy Treated test item also exhibited shifting of six out of seven hair bulbs on both days 3 and 5 compared to day 1.
Overall, the untreated test item group did not show any telogen formation; rather it exhibited catagen formation with increased hair bulb thickness on day 7 compared to day 1. However, the Consciousness Energy (The Trivedi Effect®) Treated test item significantly exhibited telogen formation i.e., promotes hair growth upto day 7 observation. Based on that, it is presumed that in this experiment the improvement of hair cell growth and development in terms of telogen formation could be due to the impact of The Trivedi Effect® - Biofield Energy Healing Treatment.
Conclusion
The experimental data showed that the untreated test item group showed 20% and 26.67% increased bulb thickness on day 5 and 7, respectively compared to day 1. Besides, the Biofield Energy Treated test item group exhibited 86%, 86%, and 100% of telogen follicle on day 3, 5, and 7, respectively compared to day 1. Overall, the Biofield Energy Treated test formulation significantly enhanced hair follicles in terms of telogen formation compared to the untreated test item group in vibrissae hair follicle organ culture cells derived from mice. In conclusion, The Trivedi Effect® - Consciousness Energy Healing Treatment might act as an effective hair growth enhancer and it can be used as a complementary and alternative treatment for the prevention of various types of skin-related disorders viz. necrotizing fasciitis, actinic keratosis, sebaceous cysts, diaper rash, decubitus ulcer etc. Besides, it might be useful to improve cell-to-cell communication, normal cell growth, cell differentiation, neurotransmission, cell cycling and proliferation, hormonal balance, skin health, immune and cardiovascular functions. Besides, it can also be utilized in various organ transplants like kidney, liver, and heart transplants, aging, hormonal imbalance, and various immune-related disease conditions such as Multiple Sclerosis, Aplastic Anemia, Ulcerative Colitis, Myasthenia Gravis, Alzheimer’s Disease, Pernicious Anemia, Dermatitis, Irritable Bowel Syndrome, Asthma, Atherosclerosis, Hashimoto Thyroiditis, Sjogren Syndrome, Hepatitis, Diverticulitis, Graves’ Disease, Dermatomyositis, Diabetes, Parkinson’s Disease, Systemic Lupus Erythematosus, stress, etc. to improve overall health and quality of life.
Acknowledgement
Authors gratefully acknowledged to Trivedi Global, Inc., Trivedi Science, Trivedi testimonials and Trivedi master wellness for their support. In addition, authors are thankful for the support of Dabur research foundation for conducting this study.
Conflict of Interest
Authors declare that there was no conflict of interest.
References
- Zhang S, Hu H, Zhang H, Liu S, Liu S, et al. (2012) Hair follicle stem cells derived from single rat vibrissa via organ culture reconstitute hair follicles in vivo. Cell Transplant 21(6): 1075-1085.
- Robinson M, Reynolds AJ, Jahoda CA (1997) Hair cycle stage of the mouse vibrissa follicle determines subsequent fiber growth and follicle behavior in vitro. J Invest Dermatol 108(4): 495-500.
- Kwon OS, Oh JK, Kim MH, Park SH, Pyo HK, et al. (2006) Human hair growth ex vivo is correlated with in vivo hair growth: Selective categorization of hair follicles for more reliable hair follicle organ culture. Arch Dermatol Res 297(8): 367-371.
- Philpott MP, Green MR, Kealey T (1990) Human hair growth in vitro. J Cell Sci 97(Pt 3): 463-471.
- Choi N, Shin S, Song SU, Sung JH (2018) Minoxidil promotes hair growth through stimulation of growth factor release from adipose-derived stem cells. Int J Mol Sci 19(3): 691.
- Lutgendorf SK, Mullen Houser E, Russell D, Degeest K, Jacobson G, et al. (2010) Preservation of immune function in cervical cancer patients during chemoradiation using a novel integrative approach. Brain Behav and Immun 24(8): 1231-1240.
- Ironson G, Field T, Scafidi F, Hashimoto M, Kumar M, et al. (1996) Massage therapy is associated with enhancement of the immune system’s cytotoxic capacity. Int J Neurosci 84(1-4): 205-217.
- Jain S, Hammerschlag R, Mills P, Cohen L, Krieger R, et al. (2015) Clinical studies of biofield therapies: Summary, methodological challenges, and recommendations. Glob Adv Health Med 4(Suppl): 58-66.
- Rubik B (2002) The biofield hypothesis: Its biophysical basis and role in medicine. J Altern Complement Med 8(6): 703-717.
- Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S (2015) The potential impact of biofield treatment on human brain tumor cells: A time-lapse video microscopy. J Integr Oncol 4: 141.
- Trivedi MK, Patil S, Shettigar H, Gangwar M, Jana S (2015) in vitro evaluation of biofield treatment on cancer biomarkers involved in endometrial and prostate cancer cell lines. J Cancer Sci Ther 7: 253-257.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Antibiogram, biochemical reactions and biotyping of biofield treated Providencia rettgeri. American Journal of Health Research 3(6): 344-351.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Antimicrobial sensitivity, biochemical characteristics and biotyping of Staphylococcus saprophyticus: An impact of biofield energy treatment. J Women’s Health Care 4: 271.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Shettigar H, et al. (2015) Antimicrobial susceptibility pattern, biochemical characteristics and biotyping of Salmonella paratyphi A: An impact of biofield treatment. Clin Microbiol 4: 215.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Antibiogram of biofield-treated Shigella boydii: Global burden of infections. Science Journal of Clinical Medicine 4: 121-126.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Evaluation of antibiogram, genotype and phylogenetic analysis of biofield treated Nocardia otitidis. Biol Syst Open Access 4(2): 143.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Charan S, et al. (2015) Phenotyping and 16S rDNA analysis after biofield treatment on Citrobacter braakii: A urinary pathogen. J Clin Med Genom 3: 129.
- Trivedi MK, Patil S, Shettigar H, Bairwa K, Jana S (2015) Spectroscopic characterization of chloramphenicol and tetracycline: An impact of biofield. Pharm Anal Acta 6: 395.
- Trivedi MK, Patil S, Shettigar H, Bairwa K, Jana S (2015) Spectroscopic characterization of biofield treated metronidazole and tinidazole. Med Chem 5: 340-344.
- Trivedi MK, Patil S, Shettigar H, Bairwa K, Jana S (2015) Effect of biofield treatment on spectral properties of paracetamol and piroxicam. Chem Sci J 6: 98.
- Trivedi MK, Branton A, Trivedi D, Shettigar H, Bairwa K, et al. (2015) Fourier transform infrared and ultraviolet-visible spectroscopic characterization of biofield treated salicylic acid and sparfloxacin. Nat Prod Chem Res 3: 186.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Morphological characterization, quality, yield and DNA fingerprinting of biofield energy treated alphonso mango (Mangifera indica L.). Journal of Food and Nutrition Sciences 3(6): 245-250.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Gangwar M, et al. (2015) Agronomic characteristics, growth analysis, and yield response of biofield treated mustard, cowpea, horse gram, and groundnuts. International Journal of Genetics and Genomics 3(6): 74-80.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Gangwar M, et al. (2015) Analysis of genetic diversity using simple sequence repeat (SSR) markers and growth regulator response in biofield treated cotton (Gossypium hirsutum L.). American Journal of Agriculture and Forestry 3(5): 216-221.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Gangwar M, et al. (2015) Evaluation of vegetative growth parameters in biofield treated bottle gourd (Lagenaria siceraria) and okra (Abelmoschus esculentus). International Journal of Nutrition and Food Sciences 4(6): 688-694.
- Trivedi MK, Tallapragada RM, Branton A, Trivedi D, Nayak G, et al. (2015) Evaluation of atomic, physical, and thermal properties of bismuth oxide powder: An impact of biofield energy treatment. American Journal of Nano Research and Applications 3(6): 94-98.
- Trivedi MK, Patil S, Nayak G, Jana S, Latiyal O (2015) Influence of biofield treatment on physical, structural and spectral properties of boron nitride. J Material Sci Eng 4: 181.
- Trivedi MK, Nayak G, Patil S, Tallapragada RM, Latiyal O, et al. (2015) Characterization of physical and structural properties of brass powder after biofield treatment. J Powder Metall Min 4(1): 134.
- Trivedi MK, Nayak G, Patil S, Tallapragada RM, Latiyal O, et al. (2015) Evaluation of biofield treatment on physical and structural properties of bronze powder. Adv Automob Eng 4: 119.
- Trivedi MK, Nayak G, Patil S, Tallapragada RM, Jana S, et al. (2015) Biofield treatment: An effective strategy to improve the quality of beef extract and meat infusion powder. J Nutr Food Sci 5: 389.
- Trivedi MK, Tallapragada RM, Branton A, Trivedi D, Nayak G, et al. (2015) Biofield treatment: A potential strategy for modification of physical and thermal properties of gluten hydrolysate and ipomoea macroelements. J Nutr Food Sci 5: 414.
- Sanders DA, Philpott MP, Kealey T (1994) Human pilosebaceous culture. Br J Dermatol 131: 166-176.
- Xu W, Fan W, Yao K (2012) Cyclosporine A stimulated hair growth from mouse vibrissae follicles in an organ culture model. J Biomed Res 26(5): 372-380.
- Ibrahim L, Wright EA (1975) The growth of rats and mice vibrissae under normal and abnormal conditions. J Embryol Exp Morphol 33(4): 831-844.
- Milner Y, Sudnik J, Filippi M, Kizoulis M, Kashgarian M, et al. (2002) Exogen, shedding phase of the hair growth cycle: Characterization of a mouse model J Invest Dermatol 119(3): 639-644.
- Müller Röver S, Handjiski B, van der Veen C, Eichmüller S, Foitzik K, et al. (2001) A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages J Invest Dermatol 117(1): 3-15.
- Inui S, Fukuzato Y, Nakajima T, Yoshikawa K, Itami S (2003) Identification of androgen-inducible TGF-beta1 derived from dermal papilla cells as a key mediator in androgenetic alopecia. J Investig Dermatol Symp Proc 8(1): 69-71.
- Gao J, DeRouen MC, Chen CH, Nguyen M, Nguyen NT, et al. (2008) Laminin-511 is an epithelial message promoting dermal papilla development and function during early hair morphogenesis. Genes Dev 22(15): 2111-2124.
- Choi SJ, Cho AR, Jo SJ, Hwang ST, Kim KH, et al. (2013) Effects of glucocorticoid on human dermal papilla cells in vitro. J Steroid Biochem Mol Biol 135: 24-29.
- Paus R, Müller Röver S, Van Der Veen C, Maurer M, Eichmüller S, et al. (1999) A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol 113(4): 523-532.
- Bang CY, Byun JW, Kang MJ, Yang BH, Song HJ, et al. (2013) Successful treatment of temporal triangular alopecia with topical minoxidil. Ann Dermatol 25(3): 387-388.
© 2019 Snehasis Jana. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






