- Submissions

Full Text
COJ Nursing & Healthcare
The Epigenetic Modification in the Pathogenesis of Skin Photoaging
Hanyi Zhang2, Yue Zhang2, Xiaoliang Tong1, Lihua Gao1, Liyang Kang1 and Jinrong Zeng1*
1Department of Dermatology, Third Xiangya Hospital, Central South University, China
2Xiangya Medical College, Central South University, China
*Corresponding author: Jinrong Zeng, Department of Dermatology, Third Xiangya Hospital, Central South University, Changsha, Hunan, China
Submission: April 14, 2020Published: April 22, 2021

ISSN: 2577-2007Volume7 Issue2
Abstract
Photoaging is a kind of skin damage induced by long-term exposure to ultraviolet radiation characterized by skin roughness, thickening, relaxation and wrinkles, local pigmentation or telangiectasia, and even tumorigenesis. Admittedly, the mechanisms of photoaging are varied and complicated mainly involving dermal extracellular matrix degradation, cell senescence and epidermal hyperplasia due to a combination of oxidative stress, inflammatory response and epigenetic modification. Epigenetic modifications study reversible and heritable changes in gene function in the absence of nuclear DNA sequence variation. Classic epigenetic events include methylation or hydroxy methylation of DNA dinucleotides, post-translational modifications of amino termini of histone proteins, and non-coding RNA expression. In this review, we introduced the pathological manifestation of skin aging and summarized the possible pathogenesis of photoaging comprehensively. In addition, we focused on the mechanisms of epigenetic contributors to skin aging impacted by UVA and UVB radiation.
Keywords: Epigenetic modification; Photoaging; DNA methylation; Histone modification; Non-coding RNAs
Introduction
Skin aging is a joint action influenced by Ultraviolet (UV) radiation damage (predominantly) combined with Visible Light (VIS) and infrared ray superimposed on so-called intrinsic and programmed aging. And its clinical manifestations include skin roughness, thickening, relaxation and wrinkles, local pigmentation or telangiectasia, and even tumorigenesis [1]. The characteristic histological changes are mainly the degeneration and degradation of dermal collagen and elastic fibers, and parts of them clustered into a mass [2]. Epidermal photoaging is predominantly attributable to UVB (290-315nm) because it’s higher in energy than UVA (315-400nm). However, the sunlight radiated on our skin is composed of 90-95% UVA (315-400nm) and 5-10% UVB (280-315nm) [3].
Thus, multiple speculations suggested that UVA had a greater impact on photoaging than UVB. Additionally, VIS (400-700nm) can be divided into red, orange, yellow, green, cyan, blue and purple light, among which red light (605-700nm) can promote cell growth, collagen synthesis and skin homeostasis, while blue light (400-450nm) inhibits cell proliferation and collagen synthesis by promoting ROS production, so blue light exposure is more harmful to the skin than red or green light [4]. Admittedly, excessive exposure to UV rays activates Matrix Metalloproteinases (MMPs), leading to the degradation of existing dermal collagen. An increase of MMPs decreases the synthesis of new collagen via reducing the Extra Cellular Matrix (ECM) exerted tension on fibroblasts attached to collagen fibers [5]. Recent studies showed the pathogenetic mechanisms of photoaging were closely related to DNA breaks [6], oxidative stress damage [7] and immune disorder [8] which referred to multiple signal pathways such as Mitogen Activated Protein Kinase (MAPK) [9], transforming growth factorβ1(TGFβ1) [10], nuclear factor-κB(NF-κB) [11] and other non-membrane dependent signaling pathway [12]. Therefore, the induction of fibroblast proliferation and collagen synthesis has become a novel target for many treatments.
Epigenetics is a discipline that does not change underlying DNA sequence while gene expression can be changed genetically, mainly including CpG island DNA methylation and its hydroxy methylation, post-translational modification of histone and non-coding RNA expression [13]. Epigenetic events often occur regularly and naturally while they are susceptible to various factors including age, environment factors,
and disease state [14]. New and ongoing research is constantly
uncovering the critical role of epigenetics in a variety of diseases.
Recent reports have suggested a critical link between UV (UVA and
UVB)-mediated epigenetic modifications and photoaging. Recently,
several studies have showed epigenetic modifications are involved
in multiple pathways in UV-induced skin injury like inducing
expression alterations in PI3K/AKT and NF-κB cell survival signaling
pathways and eventually leading to skin cancer [15-17]. Another
study reported sun exposure produced a significant trend towards
hypomethylation based on the analysis of a DNA methylation array
in sun-exposed and nonexposed skin samples [18]. Moreover,
compared with nonexposed skin, it expounded higher global
histone H3 acetylation levels in sun-exposed skin by increasing
EP300 and decreasing HDAC1 and SIRT1 expression [19]. Further
study found that overexpression of miR-101 and downregulation
of its target gene Ezh2 both induced cell senescence in the absence
of UVB irradiation [20]. This review comprehensively summarized
the underlying mechanisms by which skin aging prevails under
repeated exposure to UV radiation, and especially focused on the
epigenetic regulation mechanisms of UV radiation impact on skin
aging.
Mechanisms of Photoaging
The damage mechanisms of skin photoaging induced by UV irradiation combined with VIS includes oxidative stress, DNA damage, cell apoptosis, MMPs activation, inflammatory response, immunosuppression, the role of Advanced Glycation End Products (AGEs) and epidermal stem cell injury (Figure 1).
Figure 1: The exact damage mechanisms of skin photoaging induced by UV irradiation.
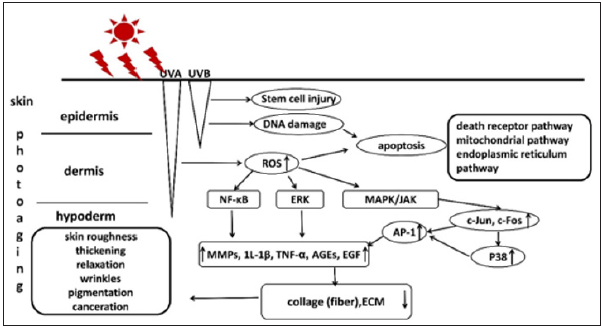
Oxidative stress
Oxidative stress is a critical cause of skin injury induced by
UVA or UVB radiation and blue light which involves an imbalance
between the ability of the body to scavenge oxygen free radicals
and the production rate of Reactive Oxygen Species (ROS) [21].
Normally, oxygen atoms bind to four electrons in the mitochondrial
respiratory chain, but single oxygen atom will carry one electron
to escape and form superoxide ions, or ROS, which will be quickly
destroyed by the skin’s antioxidant defense system. Studies have
shown that UV radiation can induce the spawn of ROS including
superoxide anion (O2
-), hydrogen peroxide (H2O2), hydroxyl
radical(·OH) and singlet oxygen(O2) [22]. When the skin is radiated
by UVB, some cell surface receptors will be activated to bind to the
corresponding ligands and stimulate the downstream signaling
molecules, leading to the activation of reduced Nicotinamide
Adenine Dinucleotide Phosphate (NADPH) and the production
of ROS [23]. Therefore, intracellular non-enzymatic antioxidant
systems (vitamin C, vitamin E, glutathione, trace elements copper,
zinc, selenium, etc.) and enzymatic antioxidant systems (superoxide
dismutase, catalase, glutathione peroxidase, etc.) are consumed by
excessive ROS, breaking the dynamic balance between oxidation
and antioxidant system in vivo [24,25]. Abnormal accumulation
of ROS in body will break intracellular biological macromolecules
(such as nucleic acid, lipids and proteins, etc.), and contribute to
abnormal activation of extracellular signal regulated kinase1/2
(ERK1/2) and NF-κB signaling pathways [26,27]. Also, it will
induce DNA damage and cell apoptosis via breaking mitochondrial
membrane potential [28].
In addition, it can promote the over-expression of Matrix Metalloproteinases (MMPs) through the Mitogen Activated Protein Kinase (MAPK) signaling pathway, and even abnormally regulate cell differentiation and proliferation to disrupt the inflammatory process, thus leading to the degradation of collagen and the occurrence of skin photoaging [29]. In fact, a recent study reported that fibroblasts exposed in blue light could also promote ROS production, which was equivalent to 25% of the total ROS production of UVA in keratinocytes [30]. Furthermore, another study showed that besides increasing ROS production, blue light radiation even reduced the expression level of per1 related to cell biological cycle rhythm, so that the repair function of cells cannot be maintained normally, thus aggravating cell damage, aging and even apoptosis [31].
DNA damage
UVB irradiation will directly or indirectly destroy DNA double strand. A variety of DNA damage in keratinocytes occurs by directly absorbing the energy of UVB, such as double-stranded DNA structure destruction, DNA chain breakage, base or base pairs excision or replacement, etc., In melanocytes or keratinocytes containing melanin experiments, UVB irradiation induces the production of Cyclobutene Pyrimidine Dimers(CPD) and pyrimidine-pyrimidone photoproducts, and then these compounds activate the protooncogenes while inactivating the tumor suppressor genes, leading to the occurrence of skin tumors [32]. Moreover, as mentioned above, UVA stimulates skin to produce a large amount of ROS, secondary inducing oxidative damage to DNA in vivo and develop cyclobutadiazine dimers, especially thymine dimmers [33]. All these variations will break single strand DNA fragments and DNA-protein crosslink, thereby hindering DNA replication and transcription, and further enhancing the carcinogenic effect of UVB [34]. In addition, blue light illuminates melanocytes to activate opsin 3 and cause the influx of calcium ions, the latter two reactions activate the ERK and P38 pathways, then activate the MITF transcription factors, which strengthens the tyrosinase activity and increases melanin synthesis, on the one hand, it causes pigment deposition and taches noir formation, on the other hand, excess ROS production induced by UV irradiation can activate the electronic in melanin, then pass the electronic energy to DNA strand to induce DNA damage, and further lead to cell apoptosis [35,36]. From this perspective, blue light also has certain effect to aggravate DNA damage and induce cell apoptosis which will further break the skin barrier function.
Cell apoptosis
UV radiation and its subsequent oxidative or DNA damage can generate mitochondrial dysfunction to induce or promote the occurrence of apoptosis [37]. Generally, the mechanisms of UV-induced apoptosis mainly include death receptor pathway, mitochondrial pathway and endoplasmic reticulum pathway. Firstly, signal transduction mediated by death receptor pathway mainly includes Fas/FasL, Tumor Necrosis Factor Receptor (TNFR) and tumor necrosis factor-related apoptosis inducing ligand signaling pathways. They trigger a series of cascade reactions during apoptosis process via different pathways [38]. Secondly, mitochondrial pathway, also known as endogenous apoptosis pathway, inhibits Bcl-2 while activating Bax, leading to mitochondrial to release cytochrome C. Then, cytochrome C unites with activator protein-1(AP-1) and Deoxyadenosine Triphosphate (DATP) to form apoptotic complex which activates caspase-9 and cleaves caspase-3. Ultimately, the activated caspase-3 further cleaves different substrates, leading to the amplification of protein cleavage cascades to induce apoptosis [39,40]. Thirdly, endoplasmic reticulum pathway can directly activate different apoptosis signal junctions such as C/EBP homologous protein pathway, P53 pathway, c-Jun aminoterminal kinase pathway, or its associated caspase pathways [41,42].
MMPs activation
MMPs are a group of zinc ion dependent internal peptidase which can specifically degrade almost all extracellular matrix in the skin. Previous studies have shown that UVA radiation can upregulate the expression of epidermal MMP-1, MMP-3 and MMP-9 [43]. Also, it has been reported that UVB radiation can induce keratinocytes to release cytokines and indirectly promote fibroblast to overexpress MMP-1by even up to 10 times by way of paracrine [44]. In addition, UVA radiation also increases the expression of transcription factors including c-Jun and c-Fos, activating c-Jun amino terminal kinase pathway and p38 mitogen activated protein kinase pathway. The latter two pathways induce the activation of AP-1 which promotes the expression of MMPs, thus stimulating the production of collagen enzyme which inhibits the synthesis of collagen and promotes its degradation [45]. Study found that infrared ray radiation also increased the expression of c-jun and reduces the production of collagen I and III in cultured human skin fibroblasts, aggravating skin aging [46].
Immunoregulatory effect
Studies have shown that UV radiation can activate the
neuroendocrine system to release neuroendocrine mediators which
increase the synthesis and secretion of multiple pro-inflammatory
cytokines in skin cells, such as histamine, serotonin and kinin
[47,48]. As mentioned above, exposure of blue light on fibroblasts
can increase ROS production, further causing cellular DNA
damage and cell senescence. Ulteriorly, study found that senescent
fibroblasts would secrete more vesicles, which were not conducive
to maintaining the function of keratinocytes in the epidermis
and increased the secretion of IL-6 [49]. These proinflammatory
mediators enhance the permeability of capillaries, leading to the
extensive infiltration and activation of neutrophils and other
phagocytes, thus contributing to skin inflammatory damage and
accelerating skin aging [50]. Additionally, UV radiation can induce
the release of pro-inflammatory cytokine interleukin 1 beta (IL-
1β) in keratinocytes which activates Epidermal Growth Factor
Receptor (EGFR) of fibroblasts and promotes the phosphorylation
of extracellular protein kinase pathways to accelerate the
degradation of collagen fiber via increasing the expression of MMP-
1 in fibroblasts which promotes the occurrence of photoaging [51].
On the other hand, UVB radiation can also stimulate keratinocytes
to generate tumor necrosis factor alpha (TNF-α) to mediate
inflammatory response [52].
Also, UVB radiation induces the expression of cyclooxygenase
2 and lipoxygenase to increase the synthesis of pro-inflammatory
mediators such as prostaglandins and thromboxins [53]. In
addition, studies showed that UVB could induce the formation
of interleukin 10(IL-10), TNF-α and other cytokines, reduce the
number of Langerhans Cells (LCS), and even affect the function of it as antigen-presenting cells to cause T cell tolerance and
suppress the skin immune system, resulting in a decline in the
body’s resistance to delayed hypersensitivity ability [54,55]. Also,
UVB irradiation induces trans-Urocanic Acid (UCA) to cis-UCA, the
latter increases the expression of Galectin-7, thereby upregulating
the proportion of apoptotic cells and inhibiting the production of
IL-2 derived from T lymphocytes, this principle is widely used in
the treatment of atopic dermatitis [56].
Age’s function
AGEs can affect the interactions between enzymes and
substrates, protein and DNA, even protein and protein, thus altering
the biological functions which are rooted in nonenzymatic reaction
products among glucose, proteins, lipids, or nucleic acids [57].
Study showed glycation in dermis generally raised after 35 years
old, then increased rapidly with intrinsic ageing [58]. Receptors for
Advanced Glycation End Product (RAGE) are extensively expressed
in the epidermis and dermis such as keratinocytes, fibroblasts,
endothelial cells and immune cells (dendritic cells, monocytes).
And it can rise when exposed to sun light which may be associated
with an increase of proinflammatory cytokine in a time-dependent
way [58]. Studies indicated in keratinocytes, AGEs influenced cell
differentiation, induced cell aging, decreased the ability of cell
vitality and migration, increased the expression of MMPs and
enhanced NF-κB signal pathway.
In Keratinocytes (KCs) culture system, they found the
expression of Involucrin (INV) and keratin 10 in normal human KCs
treated with AGE-modified collagen I or III was significantly higher
than their control group and induced the production of MMP-9 [59].
These results suggested AGE-modified collagens I and III Induce KCs
Terminal differentiation. Another study clarified the interaction of
S100A8/A9-RAGE was related with the pathogenesis of squamous
cell carcinoma in human skin [60] and their interactions aggravated
dermal fibrosis via activation of ERK1/2 MAPK and NF-kappa B
pathways in mice models [61]. Signal transduction could reduce
the proliferation of dermal fibroblasts and induce the activation of
caspass-3, caspass-8 and caspass-9 to further lead to the occurrence
of apoptosis [62,63]. Interestingly, AGEs were also found to
decrease the synthesis of collagen and extracellular matrix as well
as induce the expression of the senescence-marker β-galactosidase
[64,65]. Also, AGEs can promote the production of ROS and lower
the vitality of epidermal keratinocytes and dermal fibroblasts [66].
Epidermal stem cells injury
Epidermal stem cells are the progenitor cells of various epidermal cells. On the one hand, it can migrate downward and differentiate into epidermal basal layer, and then produce hair follicles. On the other hand, it can migrate upward and eventually differentiate into various epidermal cells, which play a critical role in repairing epidermal injury. Research has shown that UVB radiation can damage the epidermal stem cells via damaging the stem cell niche (stem cell storage site, consisting of specific extracellular matrix and niche cells) to influence the survival of epidermal stem cells and by influencing the biological rhythm of stem cells, thus inhibiting the function of stem cells to repair skin barrier [67,68]. Specifically, study reported melanoma was derived from Melanoma-Competent Melanocyte Stem Cells (MCSCs) upon stimulation by UVB. UVB induces activation and translocation of MCSC through an inflammation-dependent process. In this study, the chromatin-remodeling factor Hmga2 was identified in skin playing a key role in UVB-mediated melanoma formation. These findings delineated the potential function of MCSCs to develop melanoma following UVB stimulation [32]. Another study showed UV-irradiated endothelial cells secreted Stem Cell Factor (SCF) and increased the pigmentation of melanocytes through epithelialmesenchymal crosstalk depending on SCF/c-KIT signaling pathway during chronic sun exposure [69]. Together these results suggest that epidermal stem cells exposed upon UVB-irradiated to develop various cells to exert multiple potentials.
The Epigenetic Regulation Mechanisms of Photoaging
DNA methylation
There is still a controversial topic that UV-irradiated exposure
causes DNA methylation changes. Studies have found that longterm
exposure to UVB does not cause substantial genomic DNA
methylation changes in keratinocytes experiment in vitro, and
hypomethylation in skin cancer may be caused by inflammation
[70]. A recent study observed large blocks of hypomethylated
genome in older (over 60 years old) compared with younger subjects
(under 35 years old) in sun-exposed epidermal samples, and the
degree of hypomethylation was associated with clinical measures
of photoaging [18]. However, word explained that it would emerge
gene hypomethylation status when DNA was repairing its damage.
Therefore, more ROS production in older needs to remove, resulting
in that the body tries to initiate DNA damage repair mechanisms in
response to DNA damage and appears hypomethylated level.
However, this conjecture needs more experimental proof.
Several items have recently observed widespread distinguishable
methylated region across the genome in aging skin [71-73]. And
these data were consistent with previous report showing substantial
hypomethylation in common skin cancers such as squamous cell
carcinoma and basal cell carcinoma [74,75]. Remarkably, the overall
level of DNA methylation and the expression level of DNA methyltransferase1(
DNMT1) decreased in the process of cellular aging. A
recent study found that DNMT1 expression was markedly higher
in young Human Skin Fibroblasts (HSFs) than that in passage-aged
HSFs, and DNMT1 knockdown significantly induced the senescence
phenotype in young HSFs [76]. Nevertheless, the content of DNMT1
and Tet (DNA demethylase) was both decreased in senescent cells,
suggesting that the functions of methylation and demethylation
were also weakened [70].
Therefore, the reason for the little change of genome-wide
promoter methylation level in senescent skin cells may be related
to the specificity of gene functions. For senescent cells, some
genes such as controlling cell proliferation and differentiation will
be weakened, while genes related to cellular stress or immunity
may be enhanced [70]. Therefore, we should focus on a specific gene methylation, so as to objectively evaluate the effect of light
radiation on skin aging. The mechanism of light radiation action on
skin aging and carcinogenesis is intricate. Interestingly, cumulative
evidence identified that various DNA methylation signatures might
authenticate cell types according to their developmental potential
and possibly provide evidence for their chronological and biological
age [77,78]. Another study reported these discrepant methylation
patterns also associated with chronologically aged and photoaged
skin [79]. These data indicated large scale DNA methylation
changes involved in the onset and development of diseases induced
by environmental damage with photo-aging.
Histone modification
Histone modification plays a key role in chromatin restructuring
and the regulation of gene transcription [80]. Given previously
reported normal cellular aging was associated with global histone
modification characterized by markers H3K9me3 and H3K27me3
[81]. A recent study carried on by TG Lim et al. [82] showed Caffeic
Acid Phenethyl Ester (CAPE) could function as an epigenetic
modulator to prevent skin photoaging via targeting Histone
Acetyltransferases (HATs), and it also suppressed UV-induced global
lysine acetylation of histone H3 in both Human Dermal Fibroblasts
(HDFs) and human skin tissues [82]. Previous studies reported
that an HAT inhibitor, Anacardia Acid (AA) blocked UV-induced
MMP-1 expression and histone modifications in HDF cells through
suppressing p300 [19,83]. These studies indicate that epigenetic
regulation via inhibition of p300 can be associated with protection
from UV-mediated damages of the skin tissue. Furthermore, Ding S
et al. [84] observed a higher global histone H3 acetylation level in
sun-exposed area compared with sun-protected area, in their ChIPchip
assay, and displayed 227genes significant hyperacetylation
of histone H3 while 81 genes significant hypoacetylation of
histone H3 between the two groups. UVB irradiation regulated the
histone H3 acetylation levels by increasing EP300 expression and
decreasingHDAC1 and SIRT1 expression [19].
In addition, Sirtuin1 (SIRT1) suppressed UVB-induced p53
acetylation and its transcriptional activity, which directly affected
the cell cycle arrest. Further study on mouse demonstrated that
SIRT1 activation depressed cell senescence under UVB irradiation
[84]. Recent studies showed that the acetylated histone H3K9
levels increased at the promoters of several genes such as MMP13,
MMP12, MMP3, MMP1 and MMP10. Curiously, these findings
suggested a coordinated transcriptional activation of genes in the
MMP cluster at 11q22.3 and that acetylation of histone H3 at lysine
9 played an important role in the UVB-dependent enhancement of
transcription of MMP genes in this region [85]. Histone methylation
is also a critical modification change catalyzed by EZH2 and MLL1
enzymes [86,87]. In a skin keratinocytes study, it is mentioned
that p16INK4a gene expression increased because DNMT and
EZH2 binding in its promoter histone H3K27Me3 was decreased,
thus promoting cell senescence via inhibiting CDKs expression,
interestingly, this effect was dependent on ROS which can regulate
the methylation state through JNK-DNMT pathway [88]. In addition.
UVB has also been shown to phosphorylate histone H3 through
the p38/MSK1 pathway and stimulate COX-2 expression which
increases PGE2 level to promote cell proliferation and induce skin
cancer [89].
Non-coding RNAs
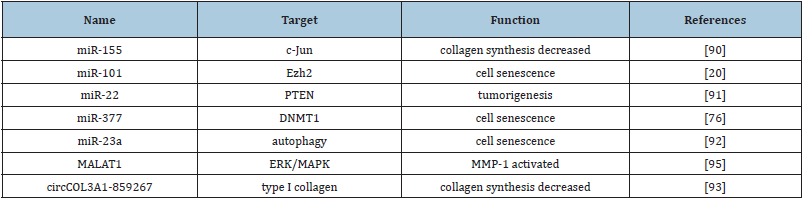
A study conducted by Greussing et al. [20] Table1 have identified
a network of miRNA-mRNA interactions mediating UVB-induced
senescence and observed a parallel activation of the p53/p21WAF1
and p16INK4a/pRb pathways [20]. Recent findings showed the
downregulation of miR-155 expressions in dermal fibroblasts
induced by UVA irradiation increased c-Jun protein and mRNA levels.
c-Jun is a critical component of transcription factor complex AP-1
which promotes the transcription of matrix metalloproteinases
to induce the degradation of extracellular matrix proteins and
negatively regulates the collagen synthesis pathway [90]. UV
irradiation-induced cellular senescence is one of the manifestations
of skin aging. miRNAs screening identified the downregulation
of miR-101 by targeting Ezh2 partially blocked the phenotype of
UVB-induced senescence [20]. MiR-22 was found to be significantly
upregulated when exposed to UVB radiation which promoted cell
survival via inhibiting the expression of tumor suppressor gene
phosphatase and tensing homolog PTEN expression. Thereby, a
long-lasting increasing level of miR-22 induced by UVB radiation
has been shown to contribute to tumorigenesis of skin cancers,
especially melanoma [91]. Another report found miR-377
induced senescence in human skin fibroblasts by targeting DNA
methyltransferase1 [76]. Furthermore, experiments demonstrated
that overexpression of miR-23a–depressed autophagy participated
in PUVA- and UVB-induced premature senescence. Abnormalities
in autophagy are associated with several pathologies, including
aging and cancer [92].
Circular RNAs (circRNAs) are a class of newly identified noncoding
RNAs with regulatory potency by sequestering miRNAs
like a sponge. A study conducted by Peng et al. [93] identified 29
significantly differentially expressed circRNAs from UVA irradiated
and no irradiated HDFs. In brief, the result showed 12 circRNAs
were up-regulated and 17 circRNAs were down-regulated.
Interestingly, they identified circCOL3A1-859267 regulate type I
collagen expression in photoaged human dermal fibroblasts which
was the most abundant proteins produced by HDFs in the dermal
collagenous extracellular matrix and decreased in photoaged skin
[93].
Furthermore, lncRNA expression profile analyzed that 1,494
lncRNAs were upregulated, and 236 lncRNAs downregulated in
the UVA-HDF group compared with the control group. Ulteriorly,
predicted lncRNA targets by bioinformatic analysis showed
correlation to MMP, cathepsin D, mitogen-activated protein
kinase and TGF-β signaling pathways [94]. Another study verified
overexpression of MALAT1 induced by UVB radiation was
independent of ROS generation and might participate in UVBinduced
photoaging by regulation of the ERK/mitogen-activated
protein kinase signaling pathway [95]. These mechanisms all
play a crucial role in human skin photoaging which suggest
abnormal expression profiles of long noncoding RNA induced by
UV-irradiation may provide novel insight to explain UV-damaging
pathology and potential targets for treatment of human skin
photoaging.
Table 1:Some non-coding RNAs action in skin photoaging included.
Conclusion
This review comprehensively summarizes the possible pathogenesis of photoaging and focus on epigenetic modification events occurring in the process of photoaging. Skin photoaging is mainly manifested in the exposed areas of sunlight. Excessive UV radiation will not only affect the appearance of the skin, but also damage human skin and accelerate skin aging. Excessive exposure to UV radiation can even cause genetic mutations and cancer. The mechanisms of photoaging refer to multiple pathways mainly embodying in the overproduction of reactive oxygen species induced by ultraviolet radiation, which result in oxidative damage of cells. Furthermore, UVB induced over-expression of MMPs destroy collagen via regulating the expression of TGF-β and AP- 1. Admittedly, as epigenetics era is coming, the transcriptional regulation and posttranslational modification in response to UV radiation has been well studied. There emerged various epigenetic changes in recent years open new horizons in well acknowledged of the molecular mechanism of ultraviolet radiation-induced skin damage. However, seeking for more effective methods to prevent and block skin photoaging is the deficiencies of the current research work, which needs further efforts in future.
Conflict of Interest
The authors declare that they have no competing interests.
References
- Han A, Chien AL, Kang S (2014) Photoaging. Dermatol Clin 32(3): 291-299.
- Gilchrest BA (2013) Photoaging. J Invest Dermatol 133(E1): E2-6.
- Battie C, Jitsukawa S, Bernerd F, Del Bino S, Marionnet C (2014) New insights in photoaging, UVA induced damage and skin types. Exp Dermatol 23(1): 7-12.
- Lorrio S, Rodriguez L, Delgado WP, Mascaraque, Gallego M, et al. (2020) Protective effect of the aqueous extract of deschampsia antarctica (edafence((r) on skin cells against blue light emitted from digital devices. Int J Mol Sci 21(3): 988.
- Poon F, Kang S, Chien AL (2015) Mechanisms and treatments of photoaging. Photodermat Photoimmunol Photomed 31(2): 65-74.
- Wong WC, Wu JY, Benzie IF (2011) Photoprotective potential of Cordyceps polysaccharides against ultraviolet B radiation-induced DNA damage to human skin cells. Br J Dermatol 164(5): 980-986.
- Kammeyer A, Luiten RM (2015) Oxidation events and skin aging. Ageing Res Rev 21: 16-29.
- Awad F, Assrawi E, Louvrier C, Jumeau C, Giurgea I, et al. (2018) Photoaging and skin cancer: is the inflammasome the missing link? Mechanisms of Ageing and Development 172: 131-137.
- Sun Z, Park SY, Hwang E, Park B, Seo SA (2016) Dietary foeniculum vulgare Mill extract attenuated UVB irradiation-induced skin photoaging by activating of Nrf2 and inhibiting MAPK pathways. Phytomedicine 23(12): 1273-1284.
- Moon NR, Kang S, Park S (2018) Consumption of ellagic acid and dihydromyricetin synergistically protects against UV-B induced photoaging, possibly by activating both TGF-beta1 and wnt signaling pathways. Journal of Photochemistry and Photobiology. Biology 178: 92-100.
- Karthikeyan R, Kanimozhi G, Prasad NR, Agilan B, Ganesan M, et al. (2016) 7-Hydroxycoumarin prevents UVB-induced activation of NF-kappaB and subsequent overexpression of matrix metalloproteinases and inflammatory markers in human dermal fibroblast cells. Journal of Photochemistry and Photobiology. Biology 161: 170-176.
- Rabe JH, Mamelak AJ, McElgunn PJ, Morison WL, Sauder DN (2006) Photoaging: mechanisms and repair. J Amer Acad Dermatol 55(1): 1-19.
- Chen Y, Hong T, Wang S, Mo J, Tian T, Zhou X (2017) Epigenetic modification of nucleic acids: from basic studies to medical applications. Chem Soc Rev 46(10): 2844-2872.
- Bishop KS (2015) The interaction between epigenetics, nutrition and the development of cancer. Nutrients 7(2): 922-947.
- Ali F, Khan BA, Sultana S (2016) Wedelolactone mitigates UVB induced oxidative stress, inflammation and early tumor promotion events in murine skin: plausible role of NFkB pathway. Eur J Pharmacol 786: 253-264.
- Gegotek A, Biernacki M, Ambrozewicz E, Surazynski A, Wronski A, et al. (2016) The cross-talk between electrophiles, antioxidant, defence and the endocannabinoid system in fibroblasts and keratinocytes after UVA and UVB irradiation. Journal of Dermatological Science 81(2): 107-117.
- Kang J, Chen W, Xia J, Li Y, Yang B, et al. (2008) Extracellular matrix secreted by senescent fibroblasts induced by UVB promotes cell proliferation in HaCaT cells through PI3K/AKT and ERK signaling pathways. Int J Mol Med 21(6): 777-784.
- Vandiver A, Irizarry RA, Hansen KD, Garza LA, Runarsson A (2015) Age and sun exposure-related widespread genomic blocks of hypomethylation in nonmalignant skin. Genome Biology 21(6): 80.
- Ding S, Chen J, Zeng Q, Lu J, Tan L, et al. (2018) Chronic sun exposure is associated with distinct histone acetylation changes in human skin. The British Journal of Dermatology 179(1): 110-117.
- Greussing R, Hackl M, Charoentong P, Pauck A, Monteforte, et al. (2013) Identification of microRNA-mRNA functional interactions in UVB-induced senescence of human diploid fibroblasts. BMC Genomics 14: 224.
- Zhang D, Lu C, Yu Z, Wang X, Yan L, et al. (2017) Echinacoside Alleviates UVB Irradiation-mediated skin damage via inhibition of oxidative stress, DNA damage, and apoptosis. Oxid Med Cell Longev 6851464.
- Jager T, Cockrell AE, Du Plessis (2017) Ultraviolet light induced generation of reactive oxygen species. Adv Exp Med Biol 996: 15-23.
- Komatsu J, Koyama H, Maeda N, Aratani Y (2006) Earlier onset of neutrophil-mediated inflammation in the ultraviolet-exposed skin of mice deficient in myeloperoxidase and NADPH oxidase. Inflam Res 55(5): 200-206.
- Xiong Y, Xiong Y, Zhou S, Sun Y, ZhaonY, Ren X, et al. (2017) Vitamin C and E supplements enhance the antioxidant capacity of erythrocytes obtained from aged rats. Rejuvenation Res 20(2): 85-92.
- Weydert CJ, Cullen JJ (2015) Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Prot 5(1): 51-66.
- Lim W, Yang C, Bazer FW (2017) Chrysophanol induces apoptosis of choriocarcinoma through regulation of ROS and the AKT and ERK1/2 pathways. Journal of Cellular Physiology 232(2): 331-339.
- Ko EY, Cho SH, Kwon SH, Eom CY, Jeong et al. (2017) The roles of NF-kappaB and ROS in regulation of pro-inflammatory mediators of inflammation induction in LPS-stimulated zebrafish embryos. Fish & Shellfish Immunology 68: 525-529.
- Song SB, Jang SY, Kang HT, Wei B, Jeoun UW, et al. (2017) Modulation of mitochondrial membrane potential and ROS generation by nicotinamide in a manner independent of SIRT1 and mitophagy. Mol Cells 40(7): 503-514.
- Park WH (2013) Effects of antioxidants and MAPK inhibitors on cell death and reactive oxygen species levels in H2O2-treated human pulmonary fibroblasts. Oncol Let 5(5): 1633-1638.
- Mann T, Eggers K, Rippke F, Tesch M, Buerger A, et al. (2019) High-energy visible light at ambient doses and intensities induces oxidative stress of skin-Protective effects of the antioxidant and Nrf2 inducer Licochalcone a in vitro and in vivo. Photodermatol Photoimmunol photomed 36(2): 135-144.
- Dong K, Goyarts EC, Pelle E, Trivero J, Pernodet N (2019) Blue light disrupts the circadian rhythm and create damage in skin cells. Int J Cos sci 41(6): 558-562.
- Moon H, Donahue LR, Choi E, Scumpia PO, Lowry WE, et al. (2017) Melanocyte stem cell activation and translocation initiate cutaneous melanoma in response to UV exposure. Cell Stem Cell 21(5): 665-678.
- Sample A, He YY (2018) Mechanisms and prevention of UV-induced melanoma. Photodermatol Photoimmunol photomed 34(1): 13-24.
- Cadet J, Wagner JR (2013) DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb perspect Biol 5(2): a012559.
- Regazzetti C, Sormani L, Debayle D, Bernerd F, Tulic MK, et al. (2018) Melanocytes sense blue light and regulate pigmentation through opsin-3. J Invest Dermatol 138(1): 171-178.
- Nakashima Y, Ohta S, Wolf AM (2017) Blue light-induced oxidative stress in live skin. Free Rad Biol Med 108: 300-310.
- Schramm H, Jaramillo ML, Quadros T, Zeni EC, Muller Y (2017) Effect of UVB radiation exposure in the expression of genes and proteins related to apoptosis in freshwater prawn embryos. Aquat Toxicol 191: 25-33.
- Jia LT, Chen SY, Yang AG (2012) Cancer gene therapy targeting cellular apoptosis machinery. Cancer Treat Rev 38 (7): 868-876.
- Zhao Y, Jing Z, Lv J (2017) Berberine activates caspase-9/cytochrome c-mediated apoptosis to suppress triple-negative breast cancer cells in vitro and in vivo. Biomed Pharmacother 95: 18-24.
- Liang S, Sun K, Wang Y, Dong S, Wang C (2016) Role of Cyt-C/caspases-9,3, Bax/Bcl-2 and the FAS death receptor pathway in apoptosis induced by zinc oxide nanoparticles in human aortic endothelial cells and the protective effect by alpha-lipoic acid. Chemico Biolog Interact 258: 40-51.
- Stevenson J, Huang EY, Olzmann JA (2016) Endoplasmic reticulum-associated degradation and lipid homeostasis. Annual Review of Nutrition 36: 511-42.
- Oakes SA (2017) Endoplasmic reticulum proteostasis: a key checkpoint in cancer. Amer J physiol 312(2): C93-c102.
- Jean C, Bogdanowicz P, Haure MJ, Castex R, Fournie JJ (2011) UVA-activated synthesis of metalloproteinases 1, 3 and 9 is prevented by a broad-spectrum sunscreen. Photodermatol Photoimmunol photomed 27(6): 318-324.
- Fagot D, Asselineau D, Bernerd F (2002) Direct role of human dermal fibroblasts and indirect participation of epidermal keratinocytes in MMP-1 production after UV-B irradiation. Archives of Dermatological Research 293(11): 576-583.
- Wang Y, Chen H, Wang W, Wang R (2014) N-terminal 5-mer peptide analog P165 of amyloid precursor protein inhibits UVA-induced MMP-1 expression by suppressing the MAPK pathway in human dermal fibroblasts. European J Pharmacol 734: 1-8.
- Liu P, Yang RL, Su H, Li LL, Song JW (2016) Expressiona of c-Jun and collagens I and III in cultured human skin fibroblasts are affected by infrared ray radiation. Journal of Southern Medical University 36(2): 163-169.
- Lupu M, Caruntu A, Caruntu C, Papagheorghe LML, Ilie MA (2017) Neuroendocrine factors: the missing link in nonmelanoma skin cancer (Review). Oncology Reports 38(3): 1327-1340.
- Han M, Ban JJ, Bae JS, Shin CY, Lee DH (2017) UV irradiation to mouse skin decreases hippocampal neurogenesis and synaptic protein expression via HPA axis activation. Scientific reports 7(1): 15574.
- Choi EJ, Kil IS, Cho EG (2020) Extracellular vesicles derived from senescent fibroblasts attenuate the dermal effect on keratinocyte differentiation. Int J Mol Sci 21(3): 1022.
- Borg M, Brincat S, Camilleri G, Schembri W, Brincat M (2013) The role of cytokines in skin aging. Climacteric: Journal of the International Menopause Society 16(5): 514-521.
- Banerjee S, Leptin M (2014) Systemic response to ultraviolet radiation involves induction of leukocytic IL-1beta and inflammation in zebrafish. Journal of Immunology 193(3): 1408-1415.
- Bernard JJ, Cowing Z, Nakatsuji T, Muehleisen B (2012) Ultraviolet radiation damages self-noncoding RNA and is detected by TLR3. Nature Medicine 18(8): 1286-1290.
- Seo SJ, Choi HG, Chung HJ, Hong CK (2002) Time course of expression of mRNA of inducible nitric oxide synthase and generation of nitric oxide by ultraviolet B in keratinocyte cell lines. British Journal of Dermatology 147(4): 655-662.
- Grandjean L, Naour R, Gangloff SC, Guenounou M (2003) Differential regulation of TNF-alpha, IL-6 and IL-10 in UVB-irradiated human keratinocytes via cyclic AMP/protein kinase a pathway. Cytokine 23(4-5): 138-149.
- Yoshiki R, Kabashima K, Sakabe J, Sugita K, Bito T, et al. (2010) The mandatory role of IL-10-producing and OX40 ligand-expressing mature Langerhans cells in local UVB-induced immunosuppression. J Immunology 184(10): 5670-5677.
- Yamaguchi T, Hiromasa K, Kabashima KR, Yoshioka M (2013) Galectin-7, induced by cis-urocanic acid and ultraviolet B irradiation, down-modulates cytokine production by T lymphocytes. Exper Dermatol 22(12): 840-842.
- Crisan M, Taulescu M, Crisan D, Cosgarea R (2013) Expression of advanced glycation end-products on sun-exposed and non-exposed cutaneous sites during the ageing process in humans. PloS One 8(10): e75003.
- Jeanmaire C (2011) Glycation during human dermal intrinsic and actinic ageing: an in vivo and in vitro model study. Br J Dermatol 145(1): 10-18.
- Fujimoto E, Kobayashi T, Fujimoto N, Akiyama M (2010) AGE-modified collagens I and III induce keratinocyte terminal differentiation through AGE receptor CD36: epidermal-dermal interaction in acquired perforating dermatosis. J Investigative Dermatology 130(2): 405-414.
- Iotzova WG, Dziunycz PJ, Freiberger SN, Lauchli S (2015) S100A8/A9 stimulates keratinocyte proliferation in the development of squamous cell carcinoma of the skin via the receptor for advanced glycation-end products. PloS One 10(3): e0120971.
- Xu X, Chen Z, Zhu X, Wang D, Liang J, et al. (2018) S100A9 aggravates bleomycin-induced dermal fibrosis in mice via activation of ERK1/2 MAPK and NF-κB pathways. Iran J Basic Med Sci 21(2): 194-201.
- Weinberg E, Maymon T, Weinreb M (2014) AGEs induce caspase-mediated apoptosis of rat BMSCs via TNF alpha production and oxidative stress. J Mol Endocr 52(1): 67-76.
- Alikhani Z, Alikhani M, Boyd CM (2005) Advanced glycation end products enhance expression of pro-apoptotic genes and stimulate fibroblast apoptosis through cytoplasmic and mitochondrial pathways. J Biolog Chem 280(13): 12087-12095.
- Zhang HB, Zhang Y, Chen C, Li YQ, Ma C (2016) Pioglitazone inhibits advanced glycation end product-induced matrix metalloproteinases and apoptosis by suppressing the activation of MAPK and NF-kappaB. Apoptosis: International Journal on Programmed Cell Death 21(10): 1082-1093.
- Ravelojaona V, Robert AM, Robert L (2009) Expression of senescence-associated beta-galactosidase (SA-beta-Gal) by human skin fibroblasts, effect of advanced glycation end-products and fucose or rhamnose-rich polysaccharides. Arch Gerontol Geriatr 48(2): 151-154.
- Mou K, Liu W, Han D (2017) HMGB1/RAGE axis promotes autophagy and protects keratinocytes from ultraviolet radiation-induced cell death. J Dermatol Sci 85(3): 162-169.
- Gong M, Zhang P, Li C, Ma X (2018) Protective mechanism of adipose-derived stem cells in remodelling of the skin stem cell niche during photoaging. Cell Physiol Biochem 51(5): 2456-2471.
- Plikus MV, Van S, Pham K, Geyfman M, Kumar V (2015) The circadian clock in skin: implications for adult stem cells, tissue regeneration, cancer, aging, and immunity. J Biol Rhythms 30(3): 163-182.
- Kim M, Shibata T, Kwon S, Park TJ (2018) Ultraviolet-irradiated endothelial cells secrete stem cell factor and induce epidermal pigmentation. Scientific Reports 8(1): 4235.
- Lahtz C (2013) UVB irradiation does not directly induce detectable changes of DNA methylation in human keratinocytes. F1000Res 2: 45.
- Ji H, Ehrlich LI, Seita J, Murakami P, Doi A, et al. (2010) Comprehensive methylome map of lineage commitment from haematopoietic progenitors. Nature 467(7313): 338-342.
- Irizarry RA, Ladd AC, Wen B, Wu Z, Montano C, et al. (2009) The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat Genet 41(2): 178-186.
- Doi A, Park IH, Wen B, Murakami P, Aryee MJ, et al. (2009) Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nat Genet 41(12): 1350-1353.
- Hansen KD, Timp W, Bravo HC, Sabunciyan S, Langmead B, et al. (2011) Increased methylation variation in epigenetic domains across cancer types. Nat Genet 43(8): 768-775.
- Berman BP, Weisenberger DJ, Aman JF, Hinoue T, Ramjan Z, et al. (2011) Regions of focal DNA hypermethylation and long-range hypomethylation in colorectal cancer coincide with nuclear lamina-associated domains. Nat Genet 44(1): 40-46.
- Xie HF, Liu YZ, Du R, Wang B, Chen MT, et al. (2017) miR-377 induces senescence in human skin fibroblasts by targeting DNA methyltransferase. Cell Death Dis 8(3): e2663.
- Horvath S (2013) DNA methylation age of human tissues and cell types. Genome Biology 14(10): R115.
- Raddatz G, Hagemann S, Aran D, Sohle J, Kulkarni, et al. (2013) Aging is associated with highly defined epigenetic changes in the human epidermis. Epigenetics Chromatin 6(1): 36.
- Gronniger E, Weber B, Heil O, Peters N, Stab F, et al. (2010) Aging and chronic sun exposure cause distinct epigenetic changes in human skin. PLoS Genetics 6(5): e1000971.
- Shankar E, Kanwal R, Candamo M, Gupta S (2016) Dietary phytochemicals as epigenetic modifiers in cancer: Promise and challenges. Semin Cancer Biol 40-41: 82-99.
- Tsurumi A, Li WX (2012) Global heterochromatin loss: a unifying theory of aging? Epigenetics 7(7): 680-688.
- Shin EJ, Jo S, Choi HK, Choi S, Byun S (2019) Caffeic acid phenethyl ester inhibits UV-induced MMP-1 expression by targeting histone acetyltransferases in human skin. Int J Mol Sci 20(12): 3055.
- Kim MK, Shin JM, Eun HC, Chung JH (2009) The role of p300 histone acetyltransferase in UV-induced histone modifications and MMP-1 gene transcription. PloS One 4(3): e4864.
- Chung KW, Choi YJ, Park MH, Jang EJ, Kim DH, et al. (2015) Molecular insights into sirt1 protection against UVB-induced skin fibroblast senescence by suppression of oxidative stress and p53 acetylation. The Journals of Gerontology 70(8): 959-968.
- Ujfaludi Z, Tuzesi A, Majoros H, Rothler B, Pankotai T, et al. (2018) Coordinated activation of a cluster of MMP genes in response to UVB radiation. Scientific Reports 8(1): 2660.
- Nutt SL, Keenan C, Chopin M, Allan RS (2019) EZH2 function in immune cell development. Biol Chem 401(8): 933-943.
- Cai S, Zhu Q, Guo C, Yuan R, Zhang X, et al. (2019) MLL1 promotes myogenesis by epigenetically regulating Myf5. Cell Prolif e12744.
- Ryu YS, Kang KA, Piao MJ, Ahn MJ, Yi JM (2019) Particulate matter-induced senescence of skin keratinocytes involves oxidative stress-dependent epigenetic modifications. Exp Mol Med 51(9): 108.
- Drouin R, Therrien JP (1997) UVB-induced cyclobutane pyrimidine dimer frequency correlates with skin cancer mutational otspots in p53. Photochem Photobiol 66(5): 719-726.
- Song J, Liu P, Yang Z, Li L, Su H (2012) MiR-155 negatively regulates c-Jun expression at the post-transcriptional level in human dermal fibroblasts in vitro: implications in UVA irradiation-induced photoaging. Cell Physiol Biochem 29(3-4): 331-340.
- Sand M, Skrygan M, Sand D, Georgas D, Gambichler T, et al. (2013) Comparative microarray analysis of microRNA expression profiles in primary cutaneous malignant melanoma, cutaneous malignant melanoma metastases, and benign melanocytic nevi. Cell Tissue Res 351(1): 85-98.
- Zhang JA, Zhou BR, Xu Y, Chen X, Liu J, et al. (2016) MiR-23a-depressed autophagy is a participant in PUVA- and UVB-induced premature senescence. Oncotarget 7(25): 37420-37435.
- Peng Y, Song X, Zheng Y, Wang X, Lai W (2017) Circular RNA profiling reveals that circCOL3A1-859267 regulate type I collagen expression in photoaged human dermal fibroblasts. Biochem Biophys Res Commun 486(2): 277-284.
- Zheng Y, Xu Q, Peng Y, Gong Z, Chen H, et al. (2017) Expression profiles of long noncoding RNA in UVA-induced human skin fibroblasts. Skin Pharmacology and Physiology 30(6): 315-323.
- Lei L, Zeng Q, Lu J, Ding S, Xia F, et al. (2017) MALAT1 participates in ultraviolet B-induced photo-aging via regulation of the ERK/MAPK signaling pathway. Mol Med Rep 15(6): 3977-3982.
© 2021 Jinrong Zeng. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






