- Submissions

Full Text
COJ Nursing & Healthcare
Improving Fulvestrant Intramuscular Injection Waiting Time for Advanced Breast Cancer Patients at Johns Hopkins Aramco Healthcare-Rapid Quality Improvement Initiative
Issam Abduljaber, RN, Salam Abou Ghaida, RN, Huda Alsayed Ahmed, PhD
1Cancer institute, Johns Hopkins Aramco Healthcare, Saudi Arabia
2Department of Quality and patient safety, Johns Hopkins Aramco Healthcare, Saudi Arabia
*Corresponding author: Huda AlsayedAhmed, PhD, CHQO, Quality and patient safety, Johns Hopkins Aramco Healthcare, Saudi Arabia
Submission: August 2, 2020;Published: September 30, 2020

ISSN: 2577-2007Volume6 Issue5
Abstract
Introduction: Fulvestrant is an estrogen receptor (ER) antagonist commonly used to treat advanced or metastatic breast cancer in postmenopausal women with hormone receptor positive. It is administered as a 1 to 2 minutes intramuscular injection designed as a solution in pre-filled syringe. Merely, placing a stock of injection medication within the ambulatory oncology clinic leans the process and makes it safer and more convenient to patient and healthcare providers. This is a quality improvement initiative; a rapid quality management PDCA cycle methodology was used. The main goal is to reduce the Fulvestrant injection wait time for Breast cancer patients by at least 60% in 3 months.
Methods and materials: Fulvestrant injections were made as a “Pyxis” item, and few doses were kept in oncology fridge to be refilled as needed and the stock is monitored. A total of 50 breast cancer cases were included in the baseline data pre-action plan implementation, a parallel number was used for post-change data analysis.
Results: Data analysis revealed that Breast cancer patient wait time reduced from 59 to 14 minutes. Ultimately, this achievement significantly reflected an increased patient satisfaction from 15% to 93% (p=0.000).
Conclusion: Certainly, inventing quality of an efficient and safe healthcare in a timely manner has clearly led to sufficient utilization of the oncology treatment rooms that accordingly has affected the patient’s satisfaction level positively.
Keywords: Fulvestrant; PDCA cycle; Efficient and safe healthcare; Patient satisfaction
Introduction
The common approaches to treat advanced or metastatic breast cancer in postmenopausal women, which are hormone-responsive tumors with progression, is relying on hindering Estrogen from binding to its estrogen receptor (ER). This is achieved by either using an anti-estrogen or reducing estrogen levels using an inhibitor [1]. Tamoxifen is an ER modulator that is broadly used hormonal treatment for breast cancer, and it is highly effective in reducing the incidence rate in high-risk patient [2], however, some patients develop resistance to it. Numerous pre-clinical studies indicated that” Livestrong” would be effective in tamoxifen-resistant breast cancer [1,3]. Fulvestrant (Faslodex) is an antineoplastic specific estrogen receptor (ER) antagonist with a steroidal novel action mode as it blocks and surges degradation of ER and thus downregulation, with no known agonist effects [4,5]. As well, when it binds to the ER it leads to a rapid loss of ER protein in the tumor ensues [4]. Consequently, this effect will result in significant reduction of tumor progesterone receptor (PgR) levels 1 therefore breast cancer suppression. Taking into consideration all the required precautions as per Food and Drug Administration (FDA) standards and other clinical guidelines, including no allergy to Fulvestrant or its ingredients. It is administered as a 1 to 2 minutes intramuscular long-acting compound for injection at the gluteal area [6].
It is designed as a 250mg/5ml solution for injection in pre-filled syringe, with no further drug concentration needed because of its low suability, which has a proven chemical-pharmaceutical quality with favorable efficacy and safety profile [7,8]. In some healthcare setting, Fulvestrant doses are located within the cancer center as one of stock items related to oncology list. Other setting might have this drug placed in the pharmacy department and releases whenever ordered by oncology center. Simply, having an injection medicine situated within the serving site, for instance ambulatory oncology clinic, makes the healthcare process safer and more convenient to patient and heath care providers.
Methods and Materials
This study conducted in an outpatient oncology institute at Johns Hopkins Aramco Healthcare (JHAH); tertiary hospital located in Kingdom of Saudi Arabia. An Ethical approval was obtained from the Institutional Review Board to publish the quality outcome of this project. This quality improvement initiative was among a list of other quality efforts to improve the service for oncology patients at JHAH. A rapid four steps quality management method; Deming PDCA cycle, was used to execute this project. The main goal is to reduce the Fulvestrant injection wait time for advanced Breast cancer patients by at least 60% in 3 months, in order to have a process that is convenient for patients and staff. This will eventually enhance patient satisfaction and mend the efficiency at oncology clinic granting sufficient use of treatment rooms. The plan (P) was to lean the process of getting, verifying and administering the Fulvestrant IM injection to Breast cancer patient in a safe and timely manner. Originally, the Fulvestrant injection was placed within the pharmacy and the order needs to be verified by pharmacist, approved, released then sent to oncology. To do (D) the proper intervention, the Fulvestrant injection was made as a “Pyxis” item, and few doses were kept in oncology fridge to be refilled as needed and the stock is monitored. Data collected after changing the practice to check (C) the performance improvement. A statistical t-Test was used to calculate the significance of the improvement. Once a progress is confirmed we acted on adjusting (A) the previous exercise accordingly.
Result
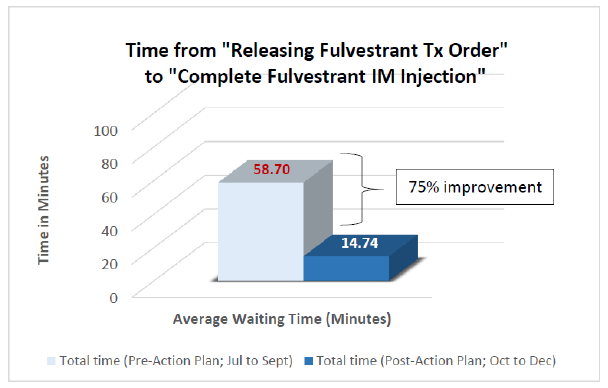
Fulvestrant Intramuscular (IM) injection Key performance indicator (KPI): This KPI defined as time from “releasing the order in Epic system” to “complete IM injection administration”. A total of 50 breast cancer cases collected for baseline data (from July to September 2018), revealed that patients wait at the oncology treatment room for almost an hour (59 minutes) to receive their Fulvestrant IM injection. Similar number of cases post implementation of action plan data collected (from October to December) showed a dramatic reduction to 14.7 minutes, with a P value ≤ 0.001 (95% Confidence Interval of 9.8 -21 minutes), with 75% improvement and time saving (Figure 1).
Figure 1: Bar graph displays the significant reduction in Fulvestrant IM injection waiting time after implementing the new practice.
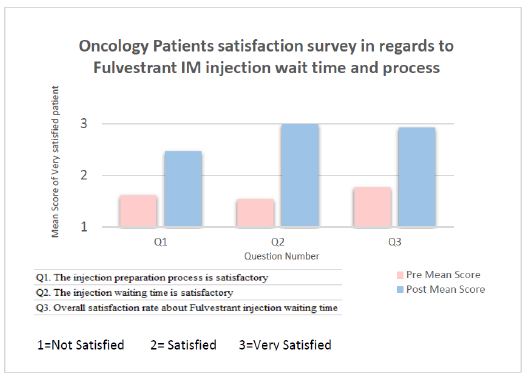
Oncology Patients Satisfaction Rate: This simple quality improvement initiative was reflected on the patient’s “Waiting time at oncology clinic” satisfaction rate. It was significantly elevated from 15% (for 3 months pre-implementation of action plan) to 93% (for 3 months post-implementation) with an obvious significant improvement (p≤0.000). This was indicated by patient’s comments collected through the monthly “Press Ganey” survey (Figure 2). Moreover, personal interview with oncology patients who received Fulvestrant treatment conducted to ensure the level of satisfaction before and after changing the process. Outcome analysis results confirmed patient satisfaction with the new practice and for spending a less time for an injection (P = 0.0004) (Figure 3).
Figure 2: Bar graph shows the significant elevation in patient satisfaction rate after changing the protocol.
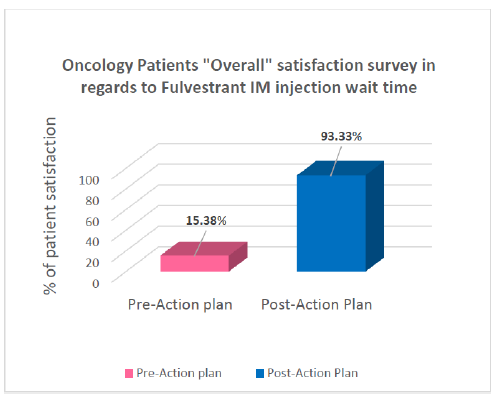
Figure 3: Bar graph illustrates the satisfaction score in relation to questionnaire-concerned points.
Discussion
Breast cancer incidence ranks first amongst other women malignancies worldwide with topmost mortality rates [9]. Fulvestrant is the anti-estrogen commonly used in clinical practice. It is the core stone of estrogen receptor (ER) pathway driven breast cancer and advanced breast cancer in postmenopausal women, as well as for hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2) naïve patients. Several clinical studies showed its significant effect yielding to longer progressionfree survival (PFS) compared to other treatments [10]. Fulvestrant commercially known as Faslodex should be administered intramuscularly, slowly, and within 1-2 minutes per injection [11]. In our organization at JHAH, this injection is kept within pharmacy department that needs to be ordered, approved and transported to oncology treatment unit. The process takes almost an hour (59 minutes) to administer the Fulvestrant IM injection to the patients. A rapid quality improvement initiative has been conducted to reduce the patient wait time for an IM injection. The post-action plan data revealed a 75% reduction in waiting time reached 14.7 minutes. In addition, this simple quality improvement project reflected an increase in patient’s satisfaction rate (from 15% to 93%) towards the “Waiting time at oncology clinic”, as indicated by the monthly “Press Ganey” survey results. Nevertheless, a reactive initiative was conducted through personal interview with breast cancer patients who received Fulvestrant treatment, seeking a feedback about the process changes. The analysis confirmed patient contentment with the new practice with a significant less time for an injection (P=0.0004).
Conclusion
In conclusion, the implemented action plan has proven to improve the Fulvestrant IM injection wait time for advanced Breast cancer patients considerably. Such improvement has also led to sufficient utilization of the oncology treatment rooms, which increased the number of served patients during the day. Certainly, inventing quality of an efficient and safe healthcare in a timely manner has clearly affected the patient’s satisfaction level positively.
References
- Lee CI, Goodwin A, Wilcken N (2017) Fulvestrant for hormone-sensitive metastatic breast cancer. Cochrane Database Syst Rev 1(1): CD011093.
- Cuzick J, Sestak I, Cawthorn S, Hamed H, Holli K, et al. (2015) Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol 16(1): 67-75.
- Robertson JFR (2007) Fulvestrant (faslodex(R)) how to make a good drug better. Oncologist 12(7): 774-784.
- Nathan MR, Schmid P (2017) A review of fulvestrant in breast cancer. Oncol Ther 5(1): 17-29.
- Kimizuka K, Inoue K, E Nagai S, Saito T, Nakano S, et al. (2019) Multicenter observational study of fulvestrant 500mg in postmenopausal Japanese women with estrogen receptor-positive advanced or recurrent breast cancer after prior endocrine treatment (SBCCSG29 Study). J Nippon Med Sch 86(3): 165-171.
- Robertson JF, Bondarenka IM, Trishkina E, Dvorkin M, Panasci L, et al. (2016) Fulvestrant 500mg versus anastrozole 1mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet 388(10063): 2997-3005.
- Deeks ED (2018) Fulvestrant: A review in advanced breast cancer not previously treated with endocrine therapy. Drugs 78(1): 131-137.
- Rocca A, Maltoni R, Bravaccini S, Donati C, Andreis D (2018) Clinical utility of fulvestrant in the treatment of breast cancer: a report on the emerging clinical evidence. Cancer Manag Res 10: 3083-3099.
- (2017) International agency for research on cancer.
- Rocca A, Maltoni R, Bravaccini S, Donati C, Andreis D (2018) Clinical utility of fulvestrant in the treatment of breast cancer: a report on the emerging clinical evidence. Cancer Manag Res 2018(10): 3083-3099.
- (2017) Injection-site pain with large-volume intramuscular injection of fulvestrant can be minimized. Oncology.
© 2020 Huda Al Sayed Ahmed. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






